New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#251 2024-09-19 15:21:30
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,272
Re: Thermal Energy Storage
I have done some back of the envelope screening calculations that tell me that TES is an expensive option if we use it to store electricity that has already been generated. The way to use this is either as: End use energy storage; or (2) To store primary heat prior to generation. The first option is how a water storage heater works. There is lots of heat using equipment that can heat as an end use. An example of the second is TES employed at a solar thermal powerplant to allow continued generation at night.
********************************************
Total costs.
1GWe steam plant: Capital cost = $1bn
3GWd(e) (7.5GWd(th)) salt storage: Capital cost = $4.5bn
Marginal annual capital cost: 10% total = $550m/year.
Operating cost = same as marginal capital cost of steam plant ($100m/year).
Power cost. Assume that half of all power generated goes into the store and 40% of what is stored is recovered. Therefore, the store consumes 0.5GWy of electricity and produces 0.2GWy. Power input cost = $0.1/kWh.
Total power consumed = 0.5 x 1000,000kW x 24 x 365.25 = 4,383,000,000kWh
Total cost = $0.1 x 4,383,000,000 = $438,300,000/year
Total cost = $1,088,300,000/year.
Power generated = 2/5 x 4,383,000,000 = 1,753,200,000kWh/year
Cost per kWh= $ 1,088,300,000/1,753,200,000 = $0.6207/kWh
For the 0.7GWy that reach the grid, cost will be:
(0.5 x 0.1 + 0.2 x 0.6207)/0.7 = $0.249/kWh.
That is expensive, considering we started with a power cost of $0.1/kWh. If the heat store can be used to store primary heat gathered from the sun, the economics are a lot more favourable. End use heat storage is better still.
Last edited by Calliban (2024-09-19 15:30:49)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#252 2024-10-22 08:18:44
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,087
Re: Thermal Energy Storage
This is of interest as it not only stores heat, but also can generate electricity, it seems.
https://www.msn.com/en-us/news/technolo … 5e88&ei=20
Quote:
New storage solution poised to revolutionize the energy sector with groundbreaking thermal technology: 'Critical to reach net-zero'
Story by Rick Kazmer • 3w • 3 min read
Even the haters of Photovoltaic, and wind, may be satisfied, if then you could perhaps use Solar Thermal with this.
Ending Pending ![]()
And this was referenced in the previous referred article: https://www.thecooldown.com/green-tech/ … rage-cool/ Quote:
New technology harnesses power of ice to scale up clean energy storage: '[It] will cost half as much as lithium-ion'
"Have you ever heard of anything safer, cleaner, and more eco-friendly than water or ice?"by Rick KazmerNovember 8, 2023
So, if I understand it they make ice when intermittant energy is available, and then use the ice for cooling purposes when it is more needed.
Pretty Clever!
Ending Pending ![]()
What happens to energy generation if you have both superheated steam from sand, and also cold stored in ice?
Could that be good on Mars?
Ending Pending ![]()
Last edited by Void (2024-10-22 08:29:35)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#253 2024-10-22 19:06:37
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,087
Re: Thermal Energy Storage
From the previous post:
And this was referenced in the previous referred article: https://www.thecooldown.com/green-tech/ … rage-cool/ Quote:
New technology harnesses power of ice to scale up clean energy storage: '[It] will cost half as much as lithium-ion'
"Have you ever heard of anything safer, cleaner, and more eco-friendly than water or ice?"by Rick KazmerNovember 8, 2023
So, if I understand it they make ice when intermittent energy is available, and then use the ice for cooling purposes when it is more needed.
Pretty Clever!
Ending Pending
What happens to energy generation if you have both superheated steam from sand, and also cold stored in ice?
Could that be good on Mars?
Ending Pending
This brings several things to mind.
Pull a vacuum on water to cool it/freeze it.
Then circulate air though to condense water out of the air.
If you are in a humid area then work with fresh water.
But if you are in an arid area use a brine tank and pull enough water vapor out of it to bring the brine temperature well below the freezing point of fresh water.
Hopefully the heat extracted could be of use to a heat pump that could produce an industrial level heat such as 180 degrees C. But then you are pulling fresh water out of the tank by using a vacuum pump.
In the old days they used to cut ice out of lakes, and store it in barns with sawdust.
Perhaps now an ice cube maker could fill a barn with ice, and then air circulated could give up water to the thaw of the ice.
For instance if you heated buildings by creating ice, then it might be possible to store ice into the summer as they did.
If ice was different, then lakes would freeze solid. If water ice sank in water that is. So, maybe if you create a vast mass of ice in such a manner then the ice mass could last seasonally.
Ending Pending ![]()
Last edited by Void (2024-10-22 19:12:50)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#254 2024-10-23 02:58:44
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,272
Re: Thermal Energy Storage
That is a useful technology. It provides a way of using intermittent solar power to provide 24/7 air conditioning and refrigeration. Building up enough ice over winter to cover summer cooling needs might be difficult. Ice stores about 92.7kWh/m3 of latent heat. It might work if you have plenty of space or an old barn as you suggest.
Calculating total cooling load required for air conditioning is complicated. This link provides guidance:
https://aircondlounge.com/cooling-load- … -examples/
The example load for a bedroom is about 3.5kW. So keeping just 1 room in a house cool for 24 hours, will melt through about 1m3 of ice. For a house, you would likely need several m3 per day.
Last edited by Calliban (2024-10-23 03:16:48)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#255 2024-10-23 08:42:05
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,087
Re: Thermal Energy Storage
Yes, seasonal does seem a stretch. I was just pondering filling an abandoned open pit mine with ice.
Generally I think cold is easier to store than hot, as long as the ground is not heated from geothermal.
I have the interest in a Heat Pump that makes ice to heat something.
I also have an interest in how such a created lump of ice or ice water could condense moisture. At night, rivers humid air may settle into a low area filled with such a coolant and deposit moisture.
A cold bath of that sort could be of brine in a tank, and you could allow or prevent the flow of air from wind to contact the cold fluid. But of course in that case you have to distill the brine to use the captured water.
Here I am thinking to allow some imagination, but before and investment it would be needed to examine the practical/economic issues later.
Ending Pending ![]()
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#256 2025-10-22 16:37:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Thermal Energy Storage
AI Overview Based on your query, here is an explanation of solar thermal power for hot water, how "watts" and "watt-hours" apply, and how to represent this information using BBCode for a forum or web page. Solar thermal power for hot water Solar thermal systems heat water directly using sunlight, rather than converting it to electricity first. Because of this, the energy output is measured in heat units, not electrical units. How it works: Solar thermal collectors (flat-plate or evacuated-tube panels) on a roof absorb solar radiation to heat a fluid. This fluid then circulates through a heat exchanger to transfer the heat to a domestic hot water tank.High efficiency: Solar thermal collectors can convert up to 80% of the sun's energy into heat for water, which is a much higher conversion rate than typical photovoltaic (PV) panels. However, this is not an apples-to-apples comparison, as PV produces electricity, which can be used for a wider variety of purposes."Watts" in context: When discussing solar thermal systems, the term "watts" (\(W\)) is used to describe the instantaneous rate of heat transfer, typically expressed as thermal kilowatts (\(kW_{th}\)). A common conversion factor is \(0.7\) \(kW_{th}\) per square meter of collector area. Electrical watts (\(W\)) are only relevant for the small amount of power needed to run pumps and system controls. Calculating and expressing energy output For solar thermal, it is most accurate to measure the total energy output over time. Watt-hours (\(Wh\)): This is a unit of energy equal to the power in watts multiplied by the time in hours. For solar thermal, this would represent the total thermal energy (\(Wh_{th}\)) produced. For example, a system with a peak thermal output of 3.5 \(kW_{th}\) that runs for 5 hours would produce \(3,500\) \(W_{th}\) * \(5\) hours = \(17,500\) \(Wh_{th}\), or \(17.5\) \(kWh_{th}\) of thermal energy.Standard ratings: The Solar Rating & Certification Corporation (ICC-SRCC) provides standard ratings for collectors in both thermal kilowatt-hours per day and thousands of BTU per day. A typical system might produce 10–15 \(kWh_{th}\) per panel per day. BBCode for formatting BBCode is a simple markup language used on many web forums. Here is how to format text related to solar thermal power calculations for a forum post. Bold: Thermal Power:Italic: Energy output can vary based on conditions.List:bbcode
Panel Type: Evacuated Tube
Peak Output: 3.5 kW[sub]th[/sub]
Total Daily Energy: 17.5 kWh[sub]th[/sub] (over 5 sun-hours)
Formatted example:bbcodeMy Solar Thermal System Performance
This is an example of the output from a solar thermal system used for hot water.
Collector Type: Evacuated Tube
Gross Area: 3.5 m[sup]2[/sup]
Rated Thermal Power: ~2.5 kW[sub]th[/sub] (Standard conditions)
Typical Daily Energy Output:
Summer (High Radiation): 15-20 kWh[sub]th[/sub]
Winter (Low Radiation): 5-10 kWh[sub]th[/sub]
AI Overview To calculate the energy output of a solar thermal hot water system, use the specific heat formula to find the thermal energy needed to heat the water. Then, account for the system's efficiency and BBCode units. Step 1: Calculate the thermal energy needed Use the specific heat formula to determine the energy required to heat a specific volume of water:Energy (in Joules) = mass (m) × specific heat of water (c) × change in temperature (ΔT) Variables: Mass (m): The mass of water you need to heat. A common conversion is 1 kilogram (kg) per liter (L) of water.Specific heat of water (\(c\)): A constant value of approximately 4.186 Joules per gram per degree Celsius (\(4186\text{\ J/kg}\degree \text{C}\)).Change in temperature (\(\Delta T\)): The desired hot water temperature minus the incoming cold water temperature, in degrees Celsius. Example calculation:To find the energy (in Joules) required to heat 100 liters of water from 10°C to 55°C: Mass (m): \(100\text{\ L}\times 1\text{\ kg/L}=100\text{\ kg}\)Specific heat (\(c\)): \(4186\text{\ J/kg}\degree \text{C}\)Change in temperature (\(\Delta T\)): \(55\degree \text{C}-10\degree \text{C}=45\degree \text{C}\) Equation:Energy (J) = \(100\text{\ kg}\times 4186\text{\ J/kg}\degree \text{C}\times 45\degree \text{C}\)Energy (J) = \(18,837,000\text{\ J}\) Step 2: Convert to Watt-hours (Wh) Energy is commonly measured in kilowatt-hours (kWh) or watt-hours (Wh). There are 3.6 million Joules in a kilowatt-hour. Convert from Joules to Watt-hours:1 Watt-hour = 3600 Joules Equation:Energy (Wh) = \(18,837,000\text{\ J}\div 3600\text{\ J/Wh}\)Energy (Wh) = \(5232.5\text{\ Wh}\) Step 3: Estimate for BBCode formatting To represent this calculation in BBCode format, which is often used on forums and is a variation of HTML, you would structure the output with bolding for emphasis, and use code blocks if available for formulas. Example BBCode: Calculating Solar Thermal Power for Hot Water
1. Calculate Thermal Energy Needed
Energy (in Joules) = mass * specific heat * temperature change
- Mass: 100kg (for 100L of water)
- Specific Heat: 4186 J/kg°C
- Temperature Change: 45°C (from 10°C to 55°C)Energy (J) = 100 * 4186 * 45 = 18,837,000 J
2. Convert to Watt-hours (Wh)
1 Wh = 3600 JEnergy (Wh) = 18,837,000 / 3600 = 5232.5 Wh
Additional factors affecting output The calculation above is for the thermal energy required, not the solar panel output. To get a more realistic estimate of the solar thermal system's performance, you must also consider: System efficiency: Real-world systems lose energy. A factor should be applied to account for these losses.Irradiation: The amount of available sunshine, which varies by location, time of day, and weather.Collector area: The size of the solar collector panels directly impacts the amount of heat absorbed.Solar fraction: The percentage of the total hot water load that the solar thermal system is expected to provide. For a more comprehensive analysis, you can use specialized software or online calculators, such as the one offered by the U.S. Department of Energ
BTU (British Thermal Unit) Calculations
1 BTU=1 DEGREE FARENHEIT INCREASE IN ONE POUND OF WATER.
8.34 POUNDS IN ONE GALLON
BTU EXAMPLE:
Incoming water 50F outgoing water 120F 120 - 50 = DELTA T 70F
BTU's for 1 gallon of water is 70 (delta T) * 8.34 (pounds per gallon) = 583.8 BTU's
Flow Rate 2 Gallons per Minute
2 Gallons * 60 minutes = 120 Gallons per hour
120 Gallons * 583.8 BTU's per Gallon = 70,056 BTU's per HourSolar Constant (measured outside the earth's atmosphere by satellite) is about 1.3 kilowatts (1,366 watts) per square meter (kW/m²)
The maximum value is about 1 Kw (1,000 watts) per sq meter, measured at the earth's exposed surface, when the sun is directly overhead.Solar Insolation Maximum sun energy (irradiance) reaching the surface of the earth over a given time period.
kWh (kiloWatt hour) to BTU examples:
1 kWh = 3,412 btu's (per square meter when the sun is directly overhead)
90,000 btu's x 0.00029307108 = 26.37 kWhCorrect Flow Rates for Evacuated Tube Collector
0.026 to 0.066 Gallons per Minute per Tube
0.1 to 0.25 Liters per Minute per TubeVolume Equivalents
231 CUBIC INCHES PER GALLON
7.481 GALLONS PER CUBIC FOOT
1.02 GALLONS PER 100' OF ½" PIPE
2.29 GALLONS PER 100' OF ¾" PIPE
4.08 GALLONS PER 100' OF 1" PIPE
Energy Conversions
1 kWh (kilo watt hours) = 3412.1415 BTU's
1 BTU = 0.00029307108 kWh
Btu Content of Common Energy Units
1 barrel (42 gallons) of crude oil = 5,800,000 Btu
1 gallon of gasoline = 124,000 Btu (based on U.S. consumption, 2008)
1 gallon of diesel fuel = 139,000 Btu
1 gallon of heating oil = 139,000 Btu
1 barrel of residual fuel oil = 6,287,000 Btu
1 cubic foot of natural gas = 1,028 Btu (based on U.S. consumption, 2008)
1 gallon of propane = 91,000 Btu
1 short ton of coal = 19,988,000 Btu (based on U.S. consumption, 2008)
1 kilowatthour of electricity = 3,412 Btu
FormulasDELTA T = TEMPERATURE DIFFERENCE BETWEEN TWO VALUES (100F-90F = 10F DELTA T)
C TO F CONVERSION (C*1.8) + 32 = F
F TO C CONVERSION (F - 32.0) / 1.8 = C
LBS * 0.453592 = KILOGRAMS
KILOGRAMS * 2.204623 = POUNDS
GALLONS * 3.785412 = LITERS
LITERS * 0.264272 = GALLONS
QUARTS * 0.946353 = LITERS
LITERS * 1.056688 = QUARTS
Offline
Like button can go here
#257 2025-10-23 09:46:24
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,350
Re: Thermal Energy Storage
According to the AI program output posted by SpaceNut, ~3.5m^2 of solar thermal collector surface area provides 17.5kWh over 5 peak generating hours per day. My surmise is that the solar insolation resource used was 1,250W/m^2:
17,500Wh / 5hrs = 3,500W
3,500W / 3.5m^2 = 1,000W/m^2
1,000W/m^2 / 0.8 (stated thermalization efficiency) = 1,250W/m^2
Houston, Texas, receives an annual average insolation of 5.27kWh/m^2/day (photonic power from the Sun), time-averaged across 4.5 to 5.5 peak insolation hours per day. We had 67 photovoltaic panels on the roof of our last home, each around 2m^2 of surface area per panel, with a BOL efficiency of 26.5%. Each 1m^2 worth of photovoltaic panel surface area therefore generated about 1,396.55Wh/m^2/day, so 187,137.7Wh/day for the entire installation. That computed value is remarkably close to the 182kWh I once saw in my wife's phone app following a sunny late July day, so I'm going to assert that using the averages for a well-sited installation is representative of objective reality.
Here's what a 134m^2 equivalent solar thermal installation would generate per day here in Houston, Texas:
5.27kWh/m^2/day * 0.8 * 134m^2 = 564,944Wh/day
That's just over 3X more power to work with. Whatever inefficiencies are associated with using that solar thermal power system, we can probably afford to eat the losses because we have some power to spare, unlike the starvation diet associated with converting all of the input photonic power from the Sun directly into electricity, at dramatically lower conversion efficiency. More importantly, we can do that without a single electrical or electronic control device, if so-desired.
Nearly all of the power consumed went directly into the home's HVAC system. All HVAC systems are giant refrigerant loops operating on the very same governing principles applicable to all other heat engines. HVAC systems don't absolutely require electrical input power, even though the vast majority of them consume electrical power to spin fans and pumps to circulate the conditioned air and refrigerant through the heat exchange loop. It's done that way because it works pretty reliably, so long as there's a continuous supply of 240V 60Hz 3-phase AC power available. Nowhere is it written that such systems absolutely must use electrical power.
Solar Thermal Air Conditioners
Solar thermal air conditioners are essentially solar water heaters that use the energy of the sun to heat up water. The hot water turns a refrigerant from liquid to gas, which absorbs heat when it condenses. The resulting cooler air is used for air conditioning, while the system also makes hot water available for household use.Solar thermal systems are more efficient than solar PV systems since it's easier to heat up and cool water than it is to produce electricity to run an electricity-powered air conditioner. This means fewer panels are needed to generate enough cooling.
This can be especially useful for roofs with a limited amount of sun exposure. However, unlike a solar PV system, you can't rely on batteries or the electrical grid to run your air conditioner at night. However, in areas where the days are hot and the nights are cool, such as in the desert, this may not be a concern.
That last part about "not being able to rely on batteries or the grid to run your AC at night" is only partially true.
"insulated hot water tank" = "batteries for a solar thermal system"
Dry sand or clay can also be used to store thermal energy for later use, perhaps most useful for larger plants located in deserts, but a hot water tank will still have the highest thermal storage capacity over the temperature ranges involved. To remove the requirement for glycol or alcohol to prevent freezing during winter, heat could be transferred using Supercritical CO2- a non-flammable thermal power transfer fluid. Aluminum heat pipes can be used instead of Copper tubing in all but high latitudes where efficiency matters most.
A diagram of a solar thermal battery system (no electrical or electronic components or grid-tie required):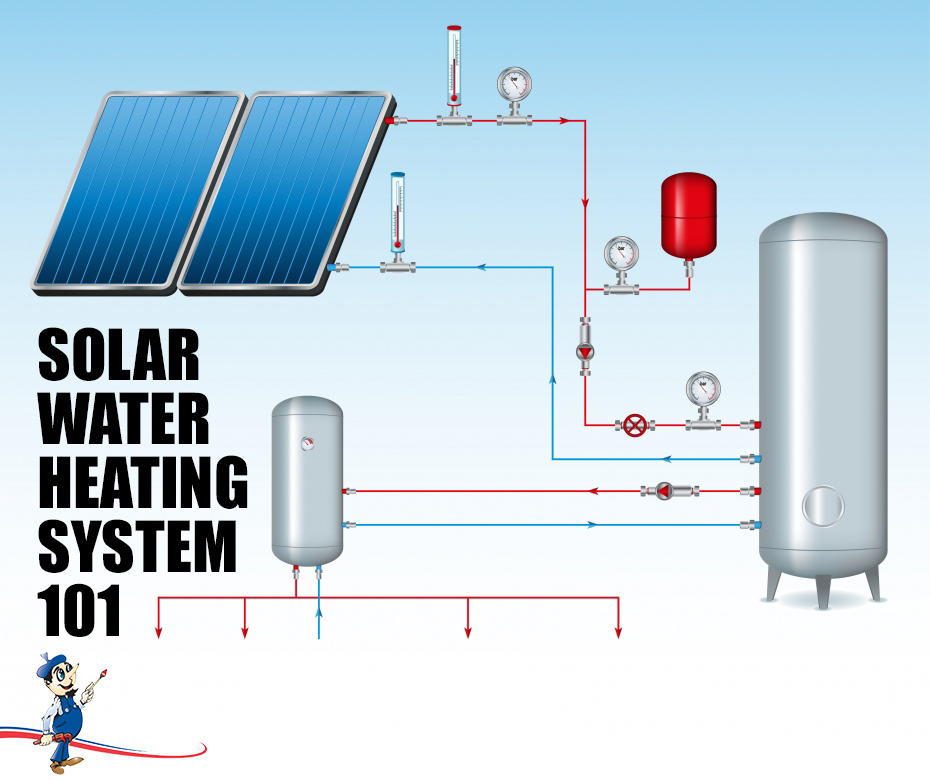
Another diagram:
A link to a commercial solar thermal water heater equipment supplier / installer:
Custom Solar and Leisure - Solar Thermal Hot Water Heater
Solaray's current home solar thermal hot water heater system was originally certified for home installation on March 23, 1993- over 32 years ago. This sort of tech was first commercialized in the 1970s. I saw them on roofs in Austin, Texas, starting in the early 1980s, along with a handful of photovoltaic installs. IIRC, I also saw them in San Diego, California. More thermally efficient evacuated tube systems are also commercially available now from a variety of other suppliers, but not too common in warmer climates where their improved efficiency is not necessary. That said, evacuated tube systems work in any climate here on Earth, on the moon (without the need for an expensive and heavy evacuated glass tube), and on Mars.
One of the most important features of evacuated tube solar thermal systems is that they rapidly become quite hot, even during winters at high latitudes. The ambient temperature can be below freezing and the sky can be overcast, but the moment the light of day kisses that tube, the temperature inside the tube will rise far above the boiling point of water (300F to 400F) in a matter of minutes. A cloud passing overhead is not going to instantly "turn the system off", either. Convection from wind, or lack thereof, also has very little effect on achieved temperature or thermal efficiency. A Canadian winter vs summer lowers efficiency by less than 5%, because the device effectively "traps" (based upon re-emission wavelengths) and thermalizes the photons. A bit of dust on the tube won't reduce the heat transfer to near-zero, either. These features make evacuated tubes suitable for thermal power generation on Mars, despite the fact that 50% less input power is available from the Sun. Boiling water on Mars or in the Arctic / Antarctic here on Earth is still readily achievable. Such systems are also used here on Earth to separate salt from sea water, to collect the salt for human consumption.
If reliable electrical power is no longer available, and it won't be if 70% of the grid's energy input is provided by photovoltaics and electric wind turbines, then we still need something that does the job of heating and cooling homes, providing hot showers, and cooking food without all the pointless cost and complexity presently used to deliver those services. Personal electronics and lights are very nice to have "optional extras" that make life much more enjoyable / entertaining. Fresh water and thermal regulation are not optional. People die without those. In the future when personal electronic devices become mostly or completely optical devices using 1M to 10M times less electrical power, the vast majority of home energy demand will still be thermal power for basic human needs and comfort.
Edit:
The National Renewable Energy Laboratory's Guide to Solar Water Heating Systems
Last edited by kbd512 (2025-10-23 09:47:32)
Offline
Like button can go here
#258 2025-10-23 14:39:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Thermal Energy Storage
Thanks, kbd512...

300 Liter Duda Solar Water Heater Active Split System Single Coil Tank Evacuated Vacuum Tubes Hot SRCC Certified
about $4,000 system
http://waterheatertimer.org/Pages/Famil … chart.html
A single tube
Concentrated solar evacuated tube lengths typically range from 1.5 to 2.1 meters for standard applications, with some specialized, high-temperature, or industrial models extending up to 3 meters. The length depends on the application, with typical lengths around 1.5 meters (\(~5\) feet) for residential solar hot water, while larger and more efficient systems used for industrial processes may feature longer tubes. Standard solar thermal collectors: These tubes are commonly around 1.5 to 2.1 meters in length (approx. \(~5\) to 7 feet).High-temperature and industrial applications: Some concentrated solar power (CSP) evacuated tubes designed for high temperatures can reach up to 3 meters (approx. \(~10\) feet) long.Manufacturer variations: The exact length will vary between manufacturers and specific product lines, so it is always best to check the product specifications.
While no standardized "200 gallon hot water tank power consumption chart" exists, you can calculate the approximate power consumption and use a table to present the data in BBCode. The actual figures vary based on the wattage of the unit, the temperature difference, and your household's hot water usage habits.Calculating power consumptionHere is the formula to calculate the power consumed over time:\((Power\ Consumption)\ (kWh)=\frac{Power\ Rating\ (Watts)\times Usage\ Time\ (Hours)}{1000}\)Key factorsWattage: A 200-gallon electric tank is not a typical residential size. It is a large, industrial-grade heater, and its wattage could be 10,000 watts (10 kW) or more. For comparison, large residential tanks (80 gallons) typically use about 4,500 watts.Usage time: This refers to the number of hours the heating elements are actively drawing power, not the full 24 hours in a day. It varies depending on how much hot water is used and the temperature setting.Example calculationFor the following chart, the calculation assumes a 10,000-watt (10 kW) unit with an average daily run time of 3 hours.Daily consumption: \((10\ kW\times 3\ hours)=30\ kWh\)Monthly consumption: \((30\ kWh\times 30\ days)=900\ kWh\)Yearly consumption: \((900\ kWh\times 12\ months)=10,800\ kWh\)Power consumption table (BBCode)bbcode200 Gallon Hot Water Tank - Estimated Power Consumption
(Assumes 10,000 Watt unit, 3 hours of heating per day)[table]
[tr]
[td]Time Period[/td]
[td]Approximate Power Consumption[/td]
[/tr]
[tr]
[td]Per Hour[/td]
[td]10 kWh[/td]
[/tr]
[tr]
[td]Per Day[/td]
[td]30 kWh[/td]
[/tr]
[tr]
[td]Per Month[/td]
[td]900 kWh[/td]
[/tr]
[tr]
[td]Per Year[/td]
[td]10,800 kWh[/td]
[/tr]
[/table]Note: This is an estimate. Actual power consumption depends on your unit's exact wattage, efficiency, and hot water usage
A 200-gallon electric water heater has a typical wattage of 4,000 to 6,000 watts and can consume an estimated 12 kWh per day, or 365 kWh per month, costing about \(\$36.50\) to \(\$438.00\) monthly depending on electricity rates and usage patterns. To find specific costs, use your local rate per kWh to calculate the daily, monthly, or yearly cost using the formula: \((\text{Wattage}\div 1000)\times \text{Hours\ Used}\times \text{Cost\ per\ kWh}=\text{Daily\ Cost}\). Consumption Level DailyMonthlyYearlyEstimated Wattage~4,000–6,000 watts~4,000–6,000 watts~4,000–6,000 wattsEstimated Energy Use~12 kWh~365 kWh~4,437 kWhEstimated Cost (at \(\$0.10\)/kWh)~$1.20~$36.50~$438.05Key factors affecting consumption Wattage: A larger tank generally uses more watts. Look for the specific wattage on the heater's label; a common range is 4,000 to 6,000 watts for large electric tanks.Usage hours: Water heaters are not running 24/7. A tank might cycle on for 3 to 5 hours per day.Electricity price: The cost per kilowatt-hour varies by location. Check your local utility bill for an accurate rate to get a precise cost estimate.Thermostat setting: Lowering the temperature can save energy, with a 5% to 10% reduction in energy use for every 10°F reduction in temperature.Efficiency: The model's age and insulation levels play a significant role. Newer, more energy-efficient models will consume less energy. How to calculate your specific cost Find your wattage: Check the data plate on your water heater. For a 5,000-watt heater: \((\frac{5000}{1000})=5\text{\ kW}\).Estimate daily usage: Multiply your wattage by the number of hours it runs per day, e.g., 3 hours: \(5\text{\ kW}\times 3\text{\ hours}=15\text{\ kWh}\) per day.Calculate daily cost: Multiply daily usage by your local rate. If your rate is \(\$0.15\) per kWh: \(15\text{\ kWh}\times \$0.15=\$2.25\) per day.Calculate monthly cost: Multiply the daily cost by 30: \(\$2.25\times 30=\$67.50\) per mont
Offline
Like button can go here
#259 2025-10-26 14:43:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Thermal Energy Storage
The red tank is the expansion tank and sizing is due to pressure after the fluid is warmed.
AI Overview
To size a solar thermal expansion tank, first calculate the total volume of the solar loop, including pipes, collectors, and heat exchanger. Next, determine the expected thermal expansion of the fluid, which is a percentage of the total volume based on the temperature difference. Finally, select a tank with an acceptance volume that is at least the calculated amount of expansion, and a total tank volume that is greater than the total system volume, using manufacturer's guidelines or a formula like the one provided by SunMaxx Solar to ensure proper pressure management.1. Calculate total system volume Add the volume of the fluid in the pipes to the volume of the solar collectors and the heat exchanger. Use manufacturer data sheets for the collectors and heat exchanger. Calculate pipe volume by multiplying the total length of the pipe runs by the fluid capacity per 100 feet (found in tables) based on pipe size and type. .CM8kHf text{fill:var(--m3c11)}.CM8kHf{font-size:1.15em}.j86kh{display:inline-block;max-width:100%} 2. Calculate the required acceptance volume This is the volume the tank needs to accommodate as the fluid expands. Fluid expansion is calculated based on the fluid type and the temperature change. For example, a common propylene glycol solution expands by slightly less than 5% between \(35^{\circ }F\) and \(200^{\circ }F\). You can use the following formula to estimate the acceptance volume: Acceptance Volume = System Volume: (\(V\)) x Coefficient of Thermal Expansion (\(\Delta T\) between max and min temp) x Safety Factor 3. Select the expansion tank Acceptance Volume: Choose a tank whose "acceptance volume" is greater than the calculated acceptance volume from the previous step. Total Volume: The "total volume" of the selected tank must be greater than the total system volume you calculated in step 1. It is better to slightly oversize the tank than to undersize it. If you are unsure, consider selecting a tank one size larger than what the calculations suggest, especially in high-temperature systems.
Offline
Like button can go here