New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2025-03-08 11:33:04
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Iron Cycle on Mars and other worlds.(Materials Extraction)
If you want to consolidate this into the "Iron and Steel on Mars by JoshNH4H", we can.
Mars apparently being rusty, my notion is to start with low grade ore and then mix it with something like Algae. Algae may be possible to grow in very basic habitable environments that could be created. The algae would likely produce excess Oxygen, if being grown in sunlight.
The mix then could be subjected to Pyrolysis, to reduce the low-grade ore. This would also create H20 and CO2.
Then it may be possible to subject the solid results to a magnetic separation process and get a upgraded content of Iron, and then also tailings.
The iron rich results might be put into an impoundment of water to rust. Some CO2 might be added to facilitate rusting.
The rusting process should produce free Hydrogen.
I am not sure if the iron will be soluble or Oxidized at this point.
If possible to precipitate the iron as Oxidized, then it could be an "ore" with a higher Iron concentration than the original ore.
As Oxidized, then you could mix it again with Algae, and subject it to pyrolysis and then another magnetic separation process.
Then another rusting process.
So then going in a loop, you might generate Oxygen and Hydrogen, and eventually a beneficiated iron content ore.
I am willing to be corrected. I am a bit weak on the rusting process. I want Oxidized Iron, not soluble iron. Or rather I might like soluble iron that then precipitates into an Oxidized iron concentrate. This might mimic the ancient Earth's processes that created iron deposits.
Ending Pending ![]()
Last edited by Void (2025-03-10 08:39:26)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#2 2025-03-08 11:58:34
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,130
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Iron is needed for the human body (and other living creatures).
A topic about iron in Life Support Systems Category has the potential to accumulate knowledge about how to acquire iron for healthful living on Mars and other locations away from Earth.
This post is reserved for an index to posts that may be contributed by NewMars members.
Index:
Post #3: Selections from Google about how iron is used in the body
(th)
Offline
Like button can go here
#3 2025-03-08 12:02:23
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,130
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Because this topic about iron is placed in teh Life Support Systems category, I asked Google about how humans use iron:
Search Labs | AI Overview
Learn more
The amount of iron a person needs depends on their age, sex, and pregnancy status.
Recommended daily iron intake
9–13 years: 8 mg
14–18 years: 15 mg
19–50 years (men): 8 mg for men, 18 mg for women
51 and older: 8 mg for men and women
Pregnant women: 27 mg
Lactating women 19–30 years: 9 mg, or 10 mg for women 14–18 years
Why iron is important
Iron helps the body grow and develop.
It's needed to make hemoglobin, a protein in red blood cells that carries oxygen throughout the body.
It's also needed to make myoglobin, a protein that supplies oxygen to muscles.
Iron helps ensure that oxygen-rich blood gets to brain cells, which helps them function properly.
Iron deficiency
Iron deficiency can occur due to a diet low in iron, blood loss, or pregnancy. Symptoms include tiredness, lack of energy, shortness of breath, noticeable heartbeats, and pale skin.
Iron sources
Iron is found in animal foods like meat, poultry, and seafood. Vegetarians may need to take iron supplements because the body doesn't absorb nonheme iron in plant foods as well as heme iron in animal foods.
Iron - Consumer - NIH Office of Dietary Supplements
Aug 17, 2023 — What is iron and what does it do? Iron is a mineral that the body needs for growth and development. Your body uses iro...
NIH Office of Dietary SupplementsIron - The Nutrition Source
RDA: The Recommended Dietary Allowance (RDA) for adults 19-50 years is 8 mg daily for men, 18 mg for women, 27 mg for pregnancy, a...The Nutrition Source
Iron in Diet - UF Health
* 9 to 13 years: 8 mg/day. * 14 to 18 years: 15 mg/day. * 19 to 50 years: 18 mg/day. * 51 and older: 8 mg/day. * Pregnant women o...UF Health
Show all
This is for informational purposes only. For medical advice or diagnosis, consult a professional. Generative AI is experimental.Export
Save
Iron Requirements
The adult minimum daily requirement of iron is 1.8 mg. Only about 10 to 30 percent of the iron you consume is absorbed and used by the body. The daily requirement of iron can be achieved by taking iron supplements. Ferrous sulfate 325 mg, taken orally once a day, and by eating foods high in iron.
Hemoglobin and Functions of Iron - UCSF HealthUCSF Health
https://www.ucs
Regarding foods, Google found:
10+ Best High iron foods for anemia
https://www.pinterest.com
High iron foods for anemia — Discover recipes, home ideas, style inspiration and other ideas to try. Discover recipes...
Easy, breezy recipes · Recipes easy as 1, 2, 3 · Top 100 recipes · Tasty dinners made simple
Image from goodhousekeeping.com
Sponsored
List of Foods That Are High in Iron - 15 Iron-Rich FoodsGood Housekeeping
https://www.goodhousekeeping.com
Reviews, Recommendations, and Top Rated Products from the Editors at Good Housekeeping. Don't miss out on the best Advice & Deals of the season, reviewed by Good Housekeeping. Best of 2025.
25 Foods That Have More Iron Than Beef - 25 Foods High In Iron
Prevention
https://www.prevention.com
No red meat? No problem! These iron-rich foods will help you meet your daily intake. From Keto To The Mediterranean Diet, Experts...
Image from facty.com
Sponsored
Top 31 Foods Highest in Iron - Foods for Iron DeficiencyFacty
https://www.facty.com › foods › iron
Elevate Your Energy Levels With 31 Iron-Rich Foods. Increase Energy Levels...
18 Foods High In Iron · Top 18 Iron-Rich Foods · Best Food Sources Of Iron · Anemia Causes
Search Labs | AI Overview
Learn more
Foods High in Iron: Clams, Dark Chocolate, White Beans, and ...
Many foods are high in iron, including meat, seafood, beans, vegetables, and fortified cereals.
Meat and seafood
beef, chicken, clams, eggs, lamb, liver, oysters, pork, shrimp, and tuna.
Vegetables Broccoli, Beet greens, Collards, Spinach, and String beans.
Beans and legumes Canned or dried beans, Lentils, Peas, Tofu, and Tempeh.
Grains and cereals
bran cereals, cornmeal, enriched pasta, enriched white bread, oat cereals, and wheat products.
Other foods
Figs
Dates
Raisins
Prunes and prune juice
Dark leafy greens
Potatoes
Cabbage and Brussels sprouts
Tomato paste
The body absorbs iron from food mainly in the upper part of the small intestine. There are two types of iron in food: heme and non-heme. Heme iron is easier for the body to absorb.
Most people can get enough iron from food.
Iron-Rich Food | List of Meats And Vegetables | Red Cross Blood
Red Cross Blood Donation
52 Foods High In Iron
Mar 15, 2023Cleveland Clinic Health Essentials
Iron-Rich Foods: Sources and Supplements - WebMD
Nov 15, 2023 — How the Body Uses Iron. When you eat food with iron, iron is absorbed into your body mainly through the upper part of ...WebMD
Show all
(th)
Offline
Like button can go here
#4 2025-03-10 08:24:43
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
I ran into this: https://www.msn.com/en-us/money/other/h … 9777&ei=11
Quote:
Hydrogen plasma breakthrough could trigger emission-free metal production
Story by Aman Tripathi • 1d • 3 min read
I think it could fit in with this topic.
Where I previously suggested mixing Algae with a regolith, and then heating it, perhaps with solar heat to perform pyrolysis, I am thinking this Hydrogen Plasma process might be good for a next process to reduce the regolith of Oxygen and possibly the Carbon that might be transferred into the materials from the Algae.
Ending Pending ![]()
The process I have described here includes a "Rusting" subprocess, where Hydrogen would be produced.
I have often gone on about ice covered lakes on Mars. Of course, the ice likely needs some kind of protection from evaporation.
But the lakes could be places where a reduction of regolith process could occur which might produce free Hydrogen.
A rust might be separated using flotation with starch, to make in beneficiated concentration of Iron Oxide. That is one method possible.
But if going in a circle as I have suggested, also the rust collected, might be subjected to pyrolysis with organic materials mixed into it. Then the results of that could be magnetically separated to produce a greater iron concentrate.
The finally with sufficient concentration you might use Hydrogen Plasma as suggested in the article.
But you might also while refining Iron, deal with some type of microbial driven extraction of other substances/metals. You might do that using Oxidation or Reduction.
Ending Pending ![]()
Could we do a cycle like this on Earth? Perhaps the rusting process could be done in tanks that could be dropped deep into the ocean, where under high pressures with heat, it might occur quickly? Maybe something else. In the production of Hydrogen you could then bond it to CO2 collected.
But the rust then mixed with easy to grow biomass could be subjected to Pyrolysis, to reduce the iron or other substances. While it may seem like this would just add Carbon to the atmosphere, perhaps the Hydrogen produced could be used to make Methane from the CO2 emissions.
Anyway, we would like greenhouse gasses on Mars, most likely.
Ending Pending ![]()
Last edited by Void (2025-03-10 08:56:09)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#5 2025-03-11 09:28:14
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Simulating the way Bog Iron is formed could be a sub-process in an Ion Cycle.
https://en.wikipedia.org/wiki/Bog_iron
Quote:
Bog iron is a form of impure iron deposit that develops in bogs or swamps by the chemical or biochemical oxidation of iron carried in solution. In general, bog ores consist primarily of iron oxyhydroxides, commonly goethite (FeO(OH)).
Iron-bearing groundwater typically emerges as a spring and the iron in it forms ferric hydroxide upon encountering the oxidizing environment of the surface. Bog ore often combines goethite and magnetite, and may include vugs and stained quartz. Oxidation may occur through enzyme catalysis by iron bacteria. It is not clear whether the magnetite precipitates upon the first contact with oxygen, then oxidizes to ferric compounds, or whether the ferric compounds are reduced when exposed to anoxic conditions upon burial beneath the sediment surface and reoxidized upon exhumation at the surface.[citation needed]
In nature the process is rather slow, but perhaps it could be sped up.
Ending Pending ![]()
Last edited by Void (2025-03-11 09:30:42)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#6 2025-03-12 10:56:58
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
I really am thinking that working with Iron, biochemically may be of some value.
I would say it again; I think that regolith could be reduced by mixing it with organic matter and heating the mix in a pyrolysis oven. This would be a way to capture stored energy on a world like Mars where solar energy may be very intermittent.
In these processes it may be hoped that biology might be incorporated to possibly concentrate iron, and also to extract other materials.
A lake on Mars could be both Anaerobic on its bottom and Oxidized in its upper layers. This is true of Antarctic Dry Valley lakes.
https://en.wikipedia.org/wiki/Lake_Vanda
Quote:
Lake Vanda is a lake in Wright Valley, Victoria Land, Ross Dependency, Antarctica. The lake is 5 km (3.1 mi) long and has a maximum depth of 69 m (226 ft).[2] On its shore, New Zealand maintained Vanda Station from 1968 to 1995. At depths of greater than approximately 50 meters,[3] Lake Vanda is a hypersaline lake with a salinity more than ten times that of seawater[4] and more than the salinity of the Dead Sea. Lake Vanda is also meromictic, which means that the deeper waters of the lake don't mix with the shallower waters.[5] There are three distinct layers of water ranging in temperature from 23 °C (73 °F) on the bottom to the middle layer of 7 °C (45 °F) and the upper layer ranges from 4–6 °C (39–43 °F).[6] It is only one of the many saline lakes in the ice-free valleys of the Transantarctic Mountains.
https://en.wikipedia.org/wiki/Meromictic_lake
Quote:
A meromictic lake is a lake which has layers of water that do not intermix.[1] In ordinary, holomictic lakes, at least once each year, there is a physical mixing of the surface and the deep waters.[2]
The term meromictic was coined by the Austrian Ingo Findenegg in 1935, apparently based on the older word holomictic. The concepts and terminology used in describing meromictic lakes were essentially complete following some additions by G. Evelyn Hutchinson in 1937.[3][4][5]
A created lake could allow sunshine though it's ice layer, but that would be quite attenuated. A substitute would be a device to grow very extreme Cyano-Bacteria in sunshine.
A Meromictic Lake could store both heat and cold in it, where salt gradients allow a colder layer above a warmer layer.
If we can get organic matter from Cyano-Bacteria, that may be mixed with regolith, and subjected to pyrolysis, perhaps from concentrating mirrors or an electric oven, to reduce the regolith which we might hope will contain significant amounts of Iron.
Such reduced regolith with a hoped-for content of Iron, could be put into the warm very salty anaerobic bottom waters of a lake. A corrosion process would produce dissolved Iron, and also Hydrogen.
Reduced regolith/iron could be stored dry and only added to the lake at a rate desired. The produced Hydrogen could be reacted with Oxygen if you had it, or even perhaps with Carbon Dioxide which could be obtained from the atmosphere of Mars. That reaction could involve biology and may produce Methane.
By continuous injection of Mars atmosphere into this lake, then it is possible that the gasses Nitrogen and Argon will build up and will at saturation bubble out of the solution.
It may be possible to precipitate an Iron containing material from the anerobic water that has dissolved water in it.
Blood Falls is a current example of a possible process: https://en.wikipedia.org/wiki/Blood_Falls
Quote:
Blood Falls is an outflow of an iron(III) oxide–tainted plume of saltwater, flowing from the tongue of Taylor Glacier onto the ice-covered surface of West Lake Bonney in the Taylor Valley of the McMurdo Dry Valleys in Victoria Land, East Antarctica.
Iron-rich hypersaline water sporadically emerges from small fissures in the ice cascades. The saltwater source is a subglacial pool of unknown size overlain by about 400 metres (1,300 ft) of ice, several kilometers from its tiny outlet at Blood Falls.
Image Quote:
I think this process is similar to an earlier Earth, and also to the production of
Bog Iron.
https://en.wikipedia.org/wiki/Bog_iron
Quote:
Bog iron is a form of impure iron deposit that develops in bogs or swamps by the chemical or biochemical oxidation of iron carried in solution. In general, bog ores consist primarily of iron oxyhydroxides, commonly goethite (FeO(OH)).
Image Quote:  Quote:
Quote:
Bog ore
So, the Iron dissolved in anerobic brine might precipitate out of solution on contact with Oxygen which could be in the cold upper layers of the lake.
Oxygen could be gotten from the growth of Cyano-Bacteria, but to store it is some trouble. But we think that there is Perchlorate in the Mars soil. So, the soil could have the perchlorates and other salts washed out of it to put in the upper layers of a lake, and then the soil might be mixed with organic matter. Then the mix could be subjected to Pyrolysis, to create reduced regolith with some Iron content. I might like to see solar ovens used for the Pyrolysis, but electric ovens might be easier to use.
This could be put into the anerobic bottom of the lake to "Charge" the lake chemically.
While the Hydrogen result could be reacted with Oxygen, it might also be reacted with CO2.
Perhaps fuel cells could be run off of the fuels and Oxidizers available.
I would like to know if CO2 and Hydrogen could be reacted in a fuel cell.
Ending Pending ![]()
Last edited by Void (2025-03-12 11:32:49)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#7 2025-03-12 14:13:02
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
These are interesting ideas Void. We definitely need some creative approaches to steel production on Mars. If we have to reduce iron using electrolytically derived carbon monoxide, then each kg of molten iron requires 15.51kWh of electricity.
https://www.sciencedirect.com/science/a … 6522003460
That is about the same energy intensity as aluminium on Earth. It isn't an attractive option unless electricity is cheap. Which it won't be for while to come. Using bulk sources of biomass like blue green algae is an attractive option. It will be easy enough to dry the material by exposing it to Martian atmospheric pressure. Human waste and refuse is something that could be used as well.
Last edited by Calliban (2025-03-12 14:14:24)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#8 2025-03-12 16:48:04
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Here in this topic, I am sort of trying not to have a narrow focus, so then to attach various things to the Oxidation and Reduction of Iron. So, the device you revealed, is of interest as well.
It has now seemed to me that Iron has a bit of magic to it. It is the dividing line between Fusion and Fission, isn't it?
I am sure that there are ways to accelerate the rusting of Iron, but the process in a lake is a sort of macro machine, perhaps something like a battery.
So, then the process of the lake might give sustainability to endure dust storms, seasonal events, and I suppose machinery breakdowns.
I am also looking at the possibility that microbial mining could be in association with the major process of Oxidation or Reduction. It appears that Sulfur might be a substance to include in some cases: https://www.brunel.net/en-au/blog/minin … oorganisms
Quote:
Biomining, also known as bioleaching or microbial mining, is a process that uses microorganisms (referred to as ‘microbes’) to extract metals from ores and minerals. This approach is an alternative to traditional mining and is more environmentally friendly. The microorganisms involved in microbial mining are typically bacteria, archaea or fungi that have the ability to oxidise and solubilise metals from mineral ores.
So, then Iron might not be the only material in action. Hydrogen in the water from rusting of Iron, may give a fuel source to run the microbes that may do some action to an Oxidized or Reduced material.
The article suggests these substances could be mined:
Common metals gained from biomining:
Gold
Silver
Uranium
Nickel
Copper
Cobalt
Zinc
I would expect you to be interested in the Uranium.
While I have suggested mixing organic materials into regolith and then roasting the mix to reduce the regolith, I suppose that you could also simply add organic matter to such a lake and allow the organisms to use it against regolith.
As a practical matter, when I say lake, I probably mean more or less a canal system.
The source of organic matter could be an extreme microbe like Cyanobacteria, to grow in a minimally protected enclosure, under sunlight most likely.
But indeed, electrical organics might also do to help with some process.
In this post in another topic, I would suggest can be a source of large amounts of water to fill a canal system, while providing lots of pressurized and protected spaces: https://newmars.com/forums/viewtopic.ph … 88#p230288
Ending Pending ![]()
Last edited by Void (2025-03-12 17:04:10)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#9 2025-03-13 06:36:09
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
In biomining, we are replacing direct acid leaching with bacteria that excrete acids to do the same thing. It is an interesting approach. There are both direct and indirect methods. The direct method involves injecting the bacterìa into the ground in a solution containing their food source. The fluid is then withdrawn, ions are seperated by ion exchange membranes and the solution is reinjected along with fresh energy source (i.e acetate). In the indirect method, the bacteria are grown in a vat. They metabolise sugar or acetate producing an organic acid, which is then seperated and injected into the ground. I don't have a strong opinion of which is best. The indirect method allows bacteria to grow under more controlled conditions.
Many low grade uranium ores are mined using insitu acid leaching. So the method does come with operational experience. It will recover a lot of other metals along the way. The only problem I can see with doing this on Mars is the cold. The fluid must be heated prior to injection. Freezing point suppression with salts won't work, because brine becomes progressively more viscous at temperatures below zero. It may technically still be liquid, but its viscosity will be comparable to treacle.
Last edited by Calliban (2025-03-13 06:48:23)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#10 2025-03-13 08:18:52
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Injection is a matter of interest also.
However, I have thought that this could be more like strip mining. Perhaps not involving blasting. Just common regolith.
Step 1, would be to wash the salts out of the regolith and into a reservoir. If there are special salts like Uranium, then it may end up in this wash. Perhaps other things will be available in the salts. I am supposing that Perchlorates will also be included, so this would be toxic to humans.
Step 2, might involve pyrolysis with organic materials mixed in. I am presuming that some materials like Iron might be reduced of Oxygen.
Step 3, (Optional), do a simple magnetic separation to get a part of the regolith with more iron and a part of the regolith with less iron.
Step 4, Place the treated regolith into a water basin, such as a canal or lake. The expectation is that the regolith will react with the water itself as in rusting and possibly react with other substances put into the water. Bogs are a bit acid and anerobic, I believe. In reoxidation such as rusting, I expect Hydrogen production to be a likely result. If so, then some of the Hydrogen could be extracted from the water as a product. Or the Hydrogen might be useful to microbes.
The bottom waters likely can be anerobic, and perhaps a maximum salinity of 10 times that of the Ocean, but perhaps even fresh water.
The temperature of the water could be cold but probably would be warm, to speed up reactions.
So, the primary objective I was hoping to achieve is dissolved Iron and Hydrogen production. The environments would be toxic to humans in some cases or tolerable to humans.
One method to get the dissolved Iron out of the water would be with Perchlorates previously extracted from the regolith by washing. I am presuming that some microbe might assist with that, using the Iron as a fuel, and the Perchlorate as a Oxidizer.
But of course, there is a lot of speculation and assumption here so modes of failure are possible/likely, but some modes might be useful.
It might be that dissolved Iron could be extracted directly from the water by some other means.
Here is some science-speak about Lake Vida: https://link.springer.com/article/10.10 … 017-0346-5
Lake Vida: https://en.wikipedia.org/wiki/Lake_Vida
Quote:
The high salinity allows the brine to remain liquid at an average yearly water temperature of −13 °C (9 °F).
Quote:
Composition
Lake Vida does not possess many factors attributed to the existence of life formations. Lake Vida contains high levels of nitrous oxide (N2O) and also molecular hydrogen (H2). The chemicals are believed to be released from chemical reactions between the brine and underlying sediments. The molecular hydrogen may be crucial as an energy source for life in the lake and aids in justifying the presence of life in an oxygen-deprived environment.[9]
Quote:
Life
Scientists have found life in an Antarctic Lake Vida that was sealed off from the outside world by a thick sheet of ice several thousands of years ago.[15][16] The discovery of the ecosystem pushes the boundaries of what life can endure, and may inform the search for alien microbes on other planets, such as Mars, or on icy moons, for instance, Jupiter's moon Europa.
My hope is to be able to grow microbes also in sunlight to provide organic materials to reduce regolith with in a pyrolysis process. But I suppose that if you could extract Hydrogen from the lake waters, you could use it in the Pyrolysis to make more reduced regolith to put into the lake to which would then create more Hydrogen.
So, that would be a thermally driven biological cycle. But I believe that large greenhouses with low air pressure should be suitable to grow some photo-microbes in large quantities.
Ending Pending ![]()
It is my hope that with Oxygen derived from Perchlorates, and with Hydrogen or Methane developed in this system you could have a means to get electricity from fuel cells or Ice Engines-Generators.
This could be very helpful in dust storms, although likely on Mars nuclear will be wanted as well.
Ending Pending ![]()
Last edited by Void (2025-03-13 08:58:02)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#11 2025-03-13 11:42:26
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Well, this popped up today: https://www.msn.com/en-us/money/compani … 36fb&ei=15
Quote:
Scientists Devise Game-Changing Way to Extract Lithium From Salt Lakes
Story by Tibi Puiu • 21h • 3 min read
And this is of interest as well: Quote:
The same technology could also be used to purify water, recover valuable metals from mining wastewater, or even extract copper and other critical materials.
Maybe Uranium?
So, I suppose you would do a wash first, to get the soluble materials out of the regolith. This would likely include Perchlorates and normal salts.
Then you could pull the possible .5% magnetic Iron out of the regolith using magnetic methods.
Then you could mix it with organic mass and subject it to pyrolysis. You might extract more magnetic iron if you want to at that point or put the treated regolith into the bottom of a lake to corrode, producing Hydrogen and dissolving iron into the water.
So, it could be worth the trouble, on Mars.
Ending Pending ![]()
Last edited by Void (2025-03-13 11:51:33)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#12 2025-03-15 10:26:41
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
This could be fitted in here or could be put in other topics: 
The ideas of lighting for habitations for humans could be actively addressed by the green oval presented.
The green oval being able to move up and down in a water column might be pressurized space for humans that would also host plants, which may be helpful to the human psychology.
The dome over the lake or canal would hold a minimum differential pressure. Enough to stabilize surface water or ice. In the event that the green oval habitat would be punctured to lose pressurization, means would be inherent in the structure to facilitate the sinking of the green oval to replace lost air pressurization with water column pressurization.
Just some tricks that might be developed on Mars along with other options for hosting living things.
This should be compatible with other functions mentioned in this topic.
Ending Pending ![]()
Last edited by Void (2025-03-15 10:32:05)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#13 2025-03-16 10:25:35
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
(th) mentioned the dome in the previous post, here: https://newmars.com/forums/viewtopic.ph … 51#p230351
I am in the mood to elaborate the "Dome" and then its relation to other features it encloses and features outside of the "Dome".
Speaking about what is to be outside of the "Dome": https://en.wikipedia.org/wiki/Archimedes%27_heat_ray
Quote:
Archimedes' heat ray
Image Quote: 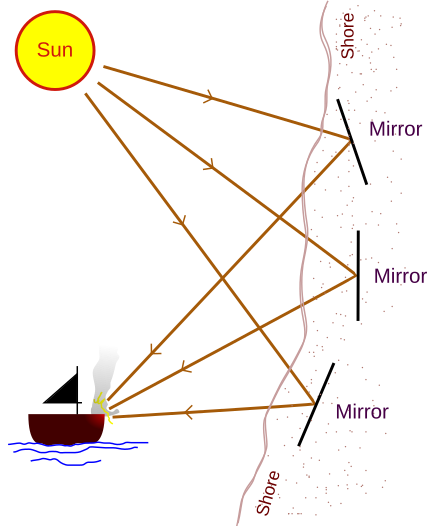
So, this "Dome" could be built mostly of Metal perhaps, with windows that Heliostats can shine light into. Also, the metal surfaces could host solar receivers of some kind so that the Heliostats could convey photon concentrations to those as an alternative. The windows might even have shutters that can cover them at times.
The "Dome" could have insulation inside of the metal parts to reduce the amount of heat loss if that is desired. That is optional to a situation and the desired consequences.
The dome would have weight but could be weighted down further to help in containing an internal air pressure that is greater than the Martian ambient pressure outside of the dome.
Pause..................
I am going to presume that by the time things like this could be built in bulk on Mars, all the easy resources for the atmosphere enhancement will have been invoked. Such as CO2 from the ice caps, and maybe even some materials absorbed in the soil, such as in clathrates. So, then optimistically an air pressure 2.5 times what it is now. This is said by some to be sufficient for true snowfalls, and temporary melt water streams.
So, perhaps instead of 5.5 mbar being the average surface air pressure, it might be 16.5 mbar. This would greatly improve the radiation situation and might allow some extreme life forms to live near the surface of Mars such as under a warm rock, maybe even some on the surface of Mars.
In this post from another topic are resources which may be helpful to the materials of this post: https://newmars.com/forums/viewtopic.ph … 13#p190313
Such as this calculator: https://endmemo.com/chem/vaporpressurewater.php
An atmospheric pressure of 15.9116 mbar would allow a vapor pressure of 14 degrees C. (57.2 degrees F). But with a dry air, evaporation may be rapid, so the dome may hold moisture in and may have a higher internal pressure than what is outside of it. 50 mbar may be sufficient to host some extreme Cyanobacteria.
But the interior of the dome might be as much as 333 mbar or even 1000 mbar, but the more pressure, I expect then the more costly it will be.
So, if you have the $$$, then you can have a habitat that suites your needs/desires.
I will do more later.
Ending Pending ![]()
Last edited by Void (2025-03-16 11:03:39)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#14 2025-03-16 11:50:21
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Continuing from posts #12 and #13 : 
Using Heliostats:
1) We can port light to the interior of the dome, for photosynthesis to occur and to make people happier.
2) We can alternately port light to a boiler.
4) We can saturate solar panels on the exterior or maybe even the interior of the dome.
3) We can do a solar or electric pyrolysis of soil and organic matter mix to produce various products including perhaps reduced regolith and reduced Iron.
In #3, depending on intentions and methods, we might vent greenhouse gasses to the Martian atmosphere, while reducing regolith.
Prior to this process we might A) Wash the salts including Perchlorates out of the regolith, B) Magnetically remove the small amount of magnetic Iron that may be available.
So, the regolith will be washed and then subjected to Pyrolysis. This should greatly reduce the toxicity of the regolith to organic life. But likely the treated regolith would be put into the bottom of the body of water inside the dome to react with the fluids. This reaction could produce Hydrogen, Dissolved Iron, maybe Lithium and Uranium as target materials to harvest.
The bottom water is expected to be anoxic and salty, at this time maybe a bit acid as to corrode, yielding the above results we might hope. Microbes might be employed in such types of mining processes as desired and possible.
The water above may be Oxygen rich and subject to sunlight for photosynthesis.
But Acetate might also be used to promote the growth of "Crops".
Here is an interesting article about Iron Oxide: https://www.msn.com/en-us/news/technolo … db20&ei=19 Quote:
Iron oxides act as natural catalysts to unlock phosphorus to fuel plant growth
Story by Amanda Morris • 1w • 5 min read
Ending Pending ![]()
Last edited by Void (2025-03-16 12:00:47)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#15 2025-03-16 20:06:30
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
While this can illustrate it is not a particularly good art: 
Here the idea would be that the arm would have flexible joints that can nevertheless be sealed well enough to keep out water. The arms might be able to allow a human to pass though.
Only a half-baked idea at this point, but maybe some sort of scissors lift might work. Granted though there can be many other ways to have habitats on Mars and other worlds.
So, the green sunroom could retract down into the water if that was a thing desired. For instance, if pressure has started to fall in the green sunroom.
But maybe this will lead to something better.
The ability to be in the sunshine is different than the desire to see Mars landscape. For landscape perhaps you might be in a bus of some sort. Or virtually explore Mars with a humanoid robot avatar.
Growing crops is then yet another thing, useful for human sanity as well as food. But it seems that crops might be able to grow off of Acetate.
The pictured sunroom might have some plants in it that would be a pleasing experience for humans.
Just a try.
Ending Pending ![]()
Last edited by Void (2025-03-16 20:13:34)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#16 2025-03-19 06:14:24
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,130
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
For Void...
This topic of yours appears to be a reasonable fit for an overview of the presence of iron on Mars. This citation includes mention of elements needed to sustain human life, which increases it's suitability since the Category is Life Support Systems.
Mars is known as the "Red Planet" due to the presence of iron oxide (rust) on its surface, which makes up a significant portion of the dust, rocks, and soil. While the exact percentage varies, it's estimated that iron oxide makes up a substantial amount of the Martian surface, contributing to its distinctive reddish hue.
Here's a more detailed explanation:
Iron Oxide Abundance:The reddish color of Mars is a result of iron oxide, or rust, that coats the surface.
Dust, Rocks, and Soil:
This iron oxide is present in the dust, rocks, and soil that cover the Martian surface.
Martian Dust:
The dust on Mars is fine, like talcum powder, and is a significant component of the surface.
Iron-Rich Mantle:
Martian meteorites suggest that the planet's mantle is about twice as rich in iron as the Earth's mantle.Oxidation:
The iron minerals in the Martian dirt oxidize, or rust, causing the surface to look red.Surface Composition:
The Martian crust consists mostly of volcanic basalt rock, with the soil also containing nutrients like sodium, potassium, chloride, and
magnesium.Mars: Facts - NASA Science
Nov 20, 2024 — Even today, it is frequently called the "Red Planet" because iron minerals in the Martian dirt oxidize, or rust, causi...NASA Science
Why Is Mars Red? The Science Behind Its Surface Colour
Sep 27, 2024 — Mars' surface is dominated by a red-orange tint visible from space. The colour is largely due to iron-oxide, also know...The Mars Society of Canada
Mars is called the Red Planet because the iron oxide (or rust ...
Feb 8, 2024 — Iron makes up 1 out of 5 parts of Mars's surface composition, which is equal to 20%. This means that 20% of the element...Brainly
Show all
Generative AI is experimental.
(th)
Offline
Like button can go here
#17 2025-03-19 07:10:04
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Void, this is interesting work. If we can find or engineer aqueous microorganisms that can extract iron oxide from a solute, it would be very valuable. Mars regolith is about 15% iron oxide on average. Producing reduced iron from this material means heating a lot of inert and useless solid as well and then removing them from reduced iron by crushing and magnetic separation. A concentrated iron oxide feedstock would save a lot of energy. A biomass material with a high percentage iron oxide would even better, because a carbon rich reducing agent is part of the source material.
Last edited by Calliban (2025-03-19 07:12:14)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#18 2025-03-19 10:35:39
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
Well, be careful I might be a Quack-Quack. If feel a bit like I am an alchemist, as really, I only have a partial grasp of what I am attempting.
We don't build bogs to make Iron ore, but bogs do make a form of it. As I go down the rabbit hole on this one, I find that Iron does a lot of strange things. As far as Iron in solutions and precipitations for solutions, I mostly find references to Iron in drinking water.
But the Bog example exists, and just vaguely mentions how Iron in the ground water upon reaching Oxygen changes to a different Oxidation which is not soluble in water. It does not say how the soluble Iron gets into the water from sediments.
For Lake Vida there is a bit more information. It can be asked about Iron in Lake Vida, or Hydrogen in Lake Vida.
Iron: https://link.springer.com/article/10.10 … 017-0346-5
Hydrogen/Nitrous Oxide: https://www.cbsnews.com/news/ancient-li … ctic-lake/
Quote:
The brine had very high levels of carbon-based compounds, the building blocks of life. It also possessed high levels of chemicals that generally react with each other, such as nitrous oxide and molecular hydrogen, suggesting they were being regularly replenished -- a surprising discovery, given how the lake was isolated for millennia from any obvious external sources of energy to help create them.
The overall chemistry of this brine suggests that chemical reactions between the water and the underlying sediment generated the reactive chemicals seen in the brine. The molecular hydrogen seen in the brine might serve as a fuel source to help support its microbial life, researchers added.
So, I am doing a presumption that if you washed the salts including the Perchlorates out of the fine soil portion of regolith and mix that with organic materials and subject it to Pyrolysis you might reduce some of the materials such as Iron. I am presuming that this could help forms of Iron to dissolve out of the result. The result may need to be crushed though, which is an extra effort, and any Carbon left behind may be hard for biology to access due to the results of Pyrolysis. A hope is that this material sprinkled on the bottom of a Lake/Canal, might both provide dissolved metals such as forms of Iron, and perhaps promote the formation of Hydrogen.
The Lake/Canal bottoms can be considerably warmer than the -13 C of Lake Vida, so the reactions if any will go faster, I hope. In such Lakes/Canals, a warm bottom water would be anoxic and covered with a cool/cold water layer above it which may be Oxidizing but might not be. This is to be accomplished using salt graduations, to disable thermal convection, mixing of water.
One thing in the back of my mind is if you can dissolve metals into a brine, can you electroplate them out of the brine? Far more is unknown on that.
So, you do the Pyrolysis thing and then crush the results and dump it into anoxic water to dissolve Iron and perhaps other things, then you might attempt to electroplate Iron out of it? But other substances might result, so I am extremely unsure.
Otherwise, a Bog simulation might provide some form of bog iron. Anoxic brine with dissolved Iron exposed to Oxygen will precipitate Iron Oxide out of it.
As for the gainful production of Hydrogen, from metals in brine, I don't know if such a reaction would be economic in volume. But though a slow process a system of lakes and canals could be very large.
Ending Pending ![]()
Electroplating with Iron????
https://www.scientificamerican.com/arti … with-iron/
Electrodeposition of Iron and Iron Alloys:
https://www.lenr-forum.com/attachment/1 … lloys-pdf/
My brain is full (Of it).
Ending Pending ![]()
Last edited by Void (2025-03-19 11:13:42)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#19 2025-03-23 22:16:50
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
Re: Iron Cycle on Mars and other worlds.(Materials Extraction)
So, I guess I will fit this in here: https://www.youtube.com/watch?v=M9rUPGfDQQ4
Quote:
Sun-Powered Machine Sucks CO2 From Air – Major Breakthrough Revealed!
The Electric Viking
https://scitechdaily.com/scientists-jus … -sunlight/
Quote:
Scientists Just Built a CO2-Eating Machine That Runs on Sunlight
By University of CambridgeFebruary 13, 202543 Comments5 Mins Read
So, who knows how productive the system is. But if it can produce a fuel, I will presume that it will also produce Oxygen.
It apparently uses UV light, unlike plants, so you don't need some UV protection to run it. It uses Water and CO2 from the atmosphere.
So, if it is productive, or can be made productive, it can produce fuel and Oxygen (I presume), and perhaps Acetate for agriculture, and it could be used in Pyrolysis to reduce regolith.
It should be a good deal almost anywhere humans might go in space, as after all we exhale CO2. The only environment I am familiar with that is Carbon deficient may be the Moon and perhaps some asteroids.
I think this could be very good on Mars, and could assist in getting Iron cycled as may be desired.
Ending Pending ![]()
Last edited by Void (2025-03-23 22:23:33)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here