New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#76 2025-02-23 14:13:16
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,072
Re: Atmospheric Separations
If you can chill the air after compression such that its temperature is 250K (-23°C), then any CO2 in the compressed air will liquefy at a pressure of 12bar.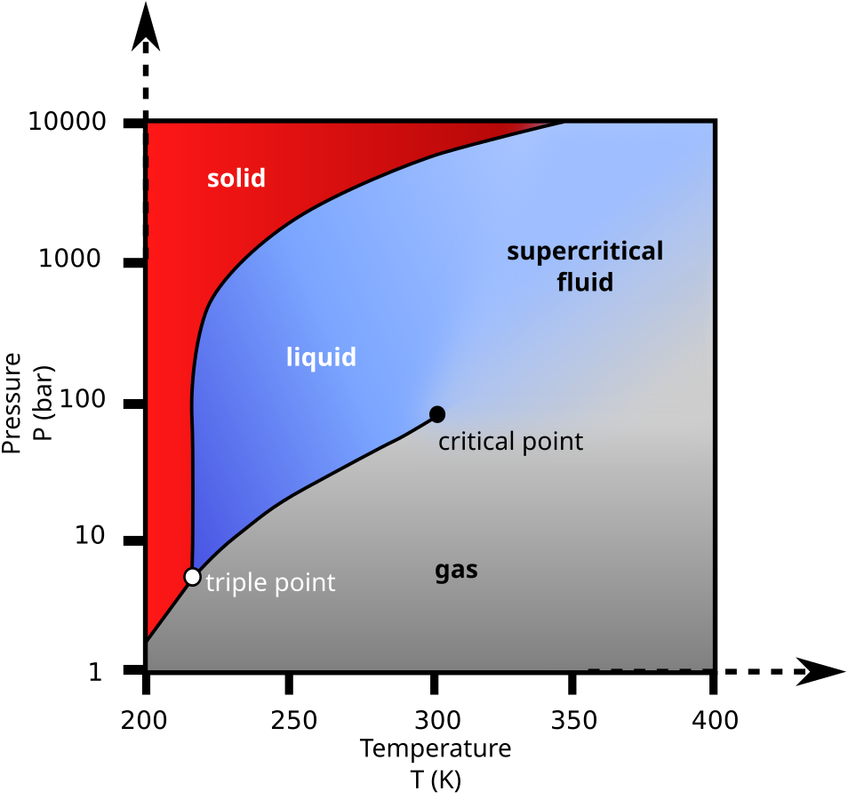
In fact, it should liquefy on the intercooler tubes of a multi-stage air compressor if pressure is taken much higher than that. To avoid building up ice on the intercooler tubes, you would want to dry the air as much as possible before compressing it. That can be done by passing it through a chiller before compression.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#77 2025-02-23 14:46:16
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,972
- Website
Re: Atmospheric Separations
Huh. So any compressed air storage system operating at high pressures will have to remove CO2 as a matter of course? Hmm... colocating storage with production and a synfuel facility as well to make use of the CO2?
Use what is abundant and build to last
Offline
Like button can go here