New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#51 2023-11-08 07:19:59
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
For RobertDyck re Post #50
Thank you for this detailed and comprehensive review of the history of a variety of propellants for rockets, including methods of shipping required atoms from Earth, and prospects for manufacture on Mars.
SearchTerm:History of rocket fuels by RobertDyck
This history will surely be required reading for anyone planning to complete in the propellant supply business on Mars, or anywhere in the Solar System.
This topic will remain focused tightly on use of glucose as the primary vehicle for shipment of atoms to locations away from Earth.
We can create as many other topics as are needed to provide a tightly focused information package for alternatives.
Because this forum has an understandable tendency to wander, it is quite normal for off-topic posts to appear from time to time.
However, ** this ** topic is intended to yield everything that an entrepreneur needs to deliver supplies to anywhere in the Solar System to preposition the ingredients customers will need when they arrive with empty tanks, with glucose as the major component, supplemented by as many additional substances as may be required.
It has been established (for example) that water is needed as part of a shipment, and it appears that dry carbon powder will help to consume excess hydrogen produced when glucose and water are fed into a conversion process to yield methane and oxygen.
In working with ChatGPT recently, I learned that SpaceX uses a ratio of 3.55:1 for rocket fuel, compared to the default of 4;1. I understand this is described as running "rich" on the methane side. Apparently there are excellent reasons to use a slightly rich mixture, including increased ISP. I gather that there is an upper limit to the use of this technique.
A post about this specific issue would be welcome in this topic. If a member decides to write such a post, please think about the audience we hope to reach, as RobertDyck has done in Post #50.
(th)
Offline
Like button can go here
#52 2023-11-08 10:27:13
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
The link below ** should ** bring up a Python program that (may) compute the progress of a chemical reaction of glucose with water and carbon to yield methane and oxygen, and an excess of methane.
https://docs.google.com/document/d/1mQ9 … sp=sharing
Here is output from version 01 of this program:
Iteration 1000: Glucose used = 180.156 g, Water used = 108.09 g, Carbon used = 12.011 g
Iteration 1000: Methane produced = 112.28 g, Oxygen produced = 191.988 g
Total mass of reactants: 300256.9999999961 g
Total mass of glucose used: 180155.99999999817 g
Total mass of water used: 108089.99999999761 g
Total mass of carbon used: 12011.000000000267 g
Total mass of methane produced: 112279.99999999905 g
Total mass of oxygen produced: 191988.00000000268 g
Ratio of methane to oxygen (by moles): 1.17:1
Extra methane (by moles): -14300.00 moles
Anyone with access to a computer running Windows, Linux or Apple can run this Python program.
Anyone with a later model Chromebook can do the same, by installing Linux, which is now an option.
The negative value for extra methane invites attention. That number should have been positive.
I ran quick totals ... the reactants total correctly, but the output is high ... I get 304268 grams for output, compared to 300257 input.
This program deserves some rework. However, it appears to be in the ball park for what is needed.
I am looking for the combination of ingredients that will total up to a ton, that will yield methane and matched oxygen, with a small excess of methane.
(th)
Offline
Like button can go here
#53 2023-11-08 10:32:01
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,421
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
Here is the electro-chemical process for making Sodium Chlorate (NaClO3), using salt (NaCl) and water (H2O) as feedstock:
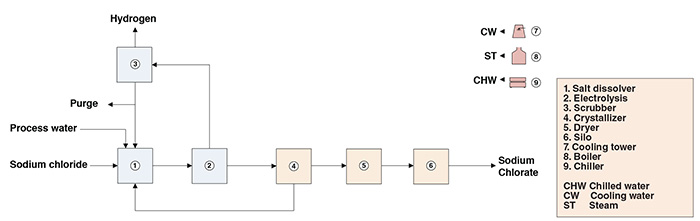
If you can obtain CO2 and H2O from Mars, then Sodium Chlorate is a storable solid white crystalline compound (much like NaCl), requiring no pressurization or temperature control. This infinitely reusable chemical will split H2 from H2O. Further heating the Sodium Chlorate (NaClO3) to 300C, after the water splitting, will then release the Oxygen.
Sodium Chlorate is used for everything from paper bleaching to cosmetics to pesticides, but the paper bleaching industry is a major user of this industrial chemical product.
That said, Hysata makes a 95%+ efficient reverse fuel cell that splits H2O. This is almost certainly the way to go. H2 from the reverse PEM fuel cell is fed into the Sabatier reactor. The Sabatier reactor combines CO2 and H2 gas into 1CH4 and 2H2O. Alternatively, CO and H2O combine into 1CH4 and 1H2O. Both types of electro-chemical reactions feed into the Sabatier process.
The primary advantage of Glucose is storability. Both Sodium Chlorate and Glucose will store indefinitely with zero pressure, irrespective of temperature (within the limits of Mars surface conditions), because they are solids.
Offline
Like button can go here
#54 2023-11-08 10:42:58
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
For kbd512 re #53
Thanks for your research on how to process the ingredients at Mars (or anywhere in the Solar System).
This process deserves it's own topic, but it is ** good ** to have it here as a first point of contact with the forum.
Can you (would you) develop this concept into a complete package that can land on Mars and go to work immediately?
This topic is primarily about shipment of ingredients for the process, but developing the machinery and systems to deliver useful outputs customers will buy is an essental part of the enterprise.
If existing manufacturers already provide everything that is needed, then the role of this forum may be to simply find them and assess their capability. Whatever equipment is needed (along with supplies) must be shipped to Mars in packages that the delivery company can handle.
NASA has set a goal of 40 tons landed gently on Mars. My understanding is that 40 tons is an aspirational goal at this point.
(th)
Offline
Like button can go here
#55 2023-11-08 12:08:51
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,421
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
We only briefly touched on an exergy analysis of propellant production, but this is absolutely critical to the success of any propellant production facility, because it dictates the cost of the equipment involved.
Hysata's capillary action reverse PEM (Proton Exchange Membrane) fuel cell (RPEMFC), requires 41.5kWh of electricity to make 1kg of H2 from H2O. H2 stores 39.4kWh per 1kg of H2 gas, when completely reacted with O2 in a 100% efficient combustion or chemical reaction process.
Every 1,000kg / 1t of H2 fuel or feedstock (for making CH4), thus requires 41.5MWh of electrical power input. The H2 content of the Starship upper stage's load of LCH4, is a bit less than 66,000kg, so 2,739MWh / 2.739GWh of electrical power is required just to create the Hydrogen. To produce this much H2 over 1 year / 8,760hrs, requires a constant power output of 312,671 Watts. This would require a massively over-sized solar array to produce 7,504,110Wh of power during just 6 hours of peak sunlight, or a Megawatt-class nuclear reactor.
On its best day, NASA's InSight lander was about to produce 4,600Wh of power from 4.2m^2 solar array. If the panels were kept clean, then 1,632 similar arrays, covering 6,854.4m^2, would be required to produce 7.5MWh of electrical power per day. An American football field covers 5,350m^2. It would probably be safe to assume that at least 2 such arrays would be required to provide the rest of the power for fuel compression and liquefaction. NASA pays about $1M/kW for these very advanced triple-junction solar arrays, so the purchase cost for 2 arrays, not the cost to ship them to Mars, is about $15B USD. For comparison purposes, the recently built 1.25GWe Watts-Bar #2 commercial nuclear reactor costs $4.7B USD to construct. This is the true cost of solar power on Mars- the electrical power to split enough Hydrogen to refuel 1 Starship would cost more than an Earth-based nuclear reactor producing 133X to 167X more power. That is why NASA has restarted its nuclear power programs. Solar power was always going to be a severe limitation. Politics killed the space nuclear programs back in the 1970s.
That is where our energy requirements start. The resultant fuel and oxidizer must then be compressed and cooled using a cryocooler.
Back in the real world, no process is ever 100% efficient. PEMFCs (Hysata's RPEMFC consumes electricity to make H2, whereas a forward or "plain old" PEMFC consumes H2 and O2 to make electricity) are typically 70% efficient. That means 27.58kWh of electricity comes out the other end of a normal PEMFC. Maybe Hysata's fuel cell could be run in reverse to produce electricity, but I doubt it. The device was deliberately engineered to be very efficient at producing H2 gas, whereas fuel cells that produce electricity are typically used in mobile applications where size / weight / power density is what matters most. Efficiency takes a backseat to raw performance. Some types of fuel cells are more efficient, some less, but PEMFC efficiency is a well-known / well-understood / highly mature technology, in use for more than 50 years. Since PEMFC operates below 100C, more common / lighter weight / lower cost materials (plastic and Aluminum) are suitable. Furthermore, the generated steam can cool them in a type of closed loop cycle. As a result, the engineering and service life of such fuel cells is something that can be accurately estimated and a wealth of knowledge available for designing newer / better / more efficient models exists. Solid Oxide fuel cells (SOFCs), which run directly on CH4 without reforming the gas into H2, are very high temperature and made from very exotic refractory metal and ceramic materials. The advantage of SOFCs is that they can readily achieve up to 85% efficiency, with greater power density than PEMFCs. The downside is that they are so hot that they can crack and they are difficult to hold onto and insulate, being as hot or hotter than the hot section of a jet engine. Heating them up or cooling them down too quickly will result in cracking and potential catastrophic failure which includes an O2/CH4 explosion.
Rocket engines, especially H2-fueled engines, typically run fuel rich to avoid melting the engine. Pure O2/H2 flame temperature is about 2,727C, which is hot enough to melt all metals commonly used in rocket engines, like the proverbial blow-torch taken to a tub of butter. Excess H2 fuel has a fantastic ability to absorb excess heat, so the extra fuel in the exhaust knocks the flame temperature back to something that is tolerable by a regeneratively cooled engine, which uses some of the H2 fuel to absorb heat from the rocket chamber / threat / nozzle before being fed into the combustion chamber or dumped overboard.
Similarly, all piston and gas turbine combustion engines also require considerable cooling to avoid melting. A very healthy portion of the generated heat is actually heating up the engine, which must be dissipated into the surrounding environment, rather than producing mechanical work output of some kind. The LH2-fueled piston inline 6-cylinder race car engine that provides electrical power and pressurization to the LH-2 fueled Centaur upper stage of the Atlas-V / Delta-IV / Vulcan families of orbital launch vehicles, powered by LH2-fueled RL-10 engine, has to be massively de-rated from 600hp+ to less than 30hp, again, to avoid melting the engine, which is cooled by H2 boil-off gas.
Either way, quite a bit of heat energy is inevitably lost. This places the overall efficiency of any process producing fuel and oxidizer, with severe mass and energy input constraints imposed by the need to ship all equipment to the surface of Mars, at an absolute premium. Efficiency is the name of the game.
Offline
Like button can go here
#56 2023-11-08 16:32:14
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
This post shows a run of the Python program to compute reactants ... the iterations was increased to 4000, and the program reached the target of one metric ton of reactants.
This program needs more work, but the output below shows that it is under control, and that it can reach the desired goal.
Shipment limit reached at iteration 3331.
Total mass of reactants: 1000156.0670000142 g
Total mass of glucose used: 600099.6360000006 g
Total mass of water used: 360047.7900000158 g
Total mass of carbon used: 40008.640999997675 g
Total mass of methane produced: 374004.6800000254 g
Total mass of oxygen produced: 639512.0280000309 g
Ratio of methane to oxygen (by moles): 1.17:1
Extra methane (by moles): -47633.30 moles
Here is the plan for the computations performed by the program that produced these results:
# React one mole of glucose to produce methane and oxygen
# The simplified reaction for glucose conversion is as follows (not a real chemical equation):
# C6H12O6 + 6H2O + C -> 7CH4 + 6O2 (This is an assumed equation for the purpose of this example)
(th)
Offline
Like button can go here
#57 2023-11-08 19:36:56
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,153
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
The calculation for a starship refill
660 mT co2 from the Atmosphere
540 mT water gathered to make
240 mT methane
960 mT oxygen
CO2 is 12 + 2(16) = 44 grams
4H2 is 4(2) = 8 grams
CH4 is 12 + 4 = 16 grams
2(H2O) is 2( 2 + 16) = 36 grams
combustion of 2 CH4 + 3 O2 ———> 2 CO2 + 4 H2O
3o2 is 3(2(16)) = 96 grams
2(CH4) is 2(12 + 4) = 32 grams
2 (co) is 2(12+8) = 40 grams
4 (H2O) is 4 ( 2+16) = 72 grams
Offline
Like button can go here
#58 2023-11-09 07:07:05
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
For SpaceNut re #57
Thank you for the timely addition of the numbers you provided, and the sugar bricks image.
Please provide the reference you used... I'm curious to see what else might be there.
Also, please take a look at this line...
combustion of 2 CH4 + 3 O2 ———> 2 CO2 + 4 H2O
I found a reference at Florida State College, showing this: CH4 + 2O2 >> CO2 + 2 H2O
Since methane has four hydrogen atoms, the number of oxygen atoms to connect with them would be even.
The equation you quoted shows an odd number of oxygen atoms, so I'm curious to know how your reference arrived at that equation.
Another reference is byjus.com ...
It shows: CH4(g) + 2O2(g) >> CO2(g) + H2O(l) + Heat
In a post after this one, RobertDyck explained that g in the notation above stands for "gas" and l stands for "liquid"
My interpretation of the situation is that the Carbon atom collects two of the available oxygen atoms, leaving two for water.
The four hydrogen atoms all go to the water, so you have the same number of atoms of each type on the left and right of the equation.
Update a bit later:
Combustion of methane and oxygen in the form of a chart:
CH4 combines with two Oxygen molecules to make one CO2 and 2 water molecules
C One carbon atom collects two oxygen atoms
H <<-- to one of the oxygen atoms
H <<-- to one of the oxygen atoms
H <<-- to the second oxygen atom
H <<-- to the second oxygen atom
O <<-- to Carbon
O <<-- to Carbon
O One oxygen atom collects two hydrogen atoms
O Second oxygen atom collects two more hydrogen atoms
(th)
Offline
Like button can go here
#59 2023-11-09 07:42:25
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,352
- Website
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
Another reference is byjus.com ...
It shows: CH4(g) + 2O2(g) >> CO2(g) + H2O(l) + Heat
I assume the g refers to grams the l to liters, but the citation does not clarify the codes.
In chemistry the (g) means gas, (l) means liquid.
Offline
Like button can go here
#60 2023-11-09 10:13:39
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
In post # 57, SpaceNut has provided the quantity of fuel and oxidizer needed to top off a Starship.
For the purposes of this topic, let's assume that the Starship that arrives at Mars is the same as the one SpaceNut cited.
A Filling Station company will want to have 1200 tons of propellant on hand, to handle any requirement, including a complete refill.
This means that (assuming a 1 ton package of Glucose, Water and Carbon) there would need to be 1200 packages shipped by slow freight to the Filling Station location on Mars.
I am inclined to suggest the Filling Station might be on Phobos.
If a customer vessel arrives at Mars in an orbit that differs significantly from that of Phobos, the needed propellant can be delivered by making the necessary plane changes using a vessel designed for the purpose. That would be done at extra charge, of course, so the wise customer would plan to arrive at Phobos for the most economical refill.
The competition is going to be using traditional two stage rocket technology to deliver propellant to Low Earth Orbit, so our enterprise is going to need to reduce costs significantly, in order to make a profit while offering customers competitive prices. I am proposing to use ballistic launch systems for this purpose.
At the moment (late in 2023) none of candidate systems are able to achieve LEO, or even to come close. However, as nearly as I can tell, there is nothing in physics that would preclude success. The problem would appear be limited to engineering (on one hand) and financing (on the other).
(th)
Offline
Like button can go here
#61 2023-11-09 14:28:51
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,819
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
After extensive sessions with ChatGPT, and with the (most welcome) assistance of NewMars members, I have discovered that the glucose idea is a dead end.
The solution arose during runs of the Python programs created by ChatGPT .... it showed that only carbon and water are needed.
In fact, the carbon powder can be dumped into a container of water (in the ratio of 1.5 water to 1 carbon) and the resulting reactant package can feed into whatever chemical processes that are needed to make methane and oxygen. Inefficiency of any process does not cause loss of reactants... they must simply be collected and fed back into the process.
This topic is hereby closed.
(th)
Offline
Like button can go here
#62 2023-11-09 19:53:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,153
Re: Glucose as Rocket Fuel/Oxidizer Shipment Method
Equations for rocket engines is not equal as the launch plume will have color to it. The color is due to rich to lean conditions of the burning of the fuel.
Also remember the propellant is also to create the needed oxygen as well from within the same processing so that the crew will have all that they need.
Offline
Like button can go here
