New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#126 2021-12-10 20:16:22
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
steam boiler energy input requirement to temperature
https://www4.eere.energy.gov/manufactur … superSteam
Steam System Modeler Tool (SSMT)
https://www.engineeringtoolbox.com/stea … d_437.html
https://www.engineeringtoolbox.com/boil … _1115.html
https://www.csemag.com/articles/getting … ign-right/
https://www.power-eng.com/coal/steam-ge … ency/#gref
Steam Generator Efficiency
https://power.mhi.com/products/conventional
Under normal atmospheric pressure [0.101 MPa], water boils at 100ºC. As pressure increases, the boiling temperature of water also increases. When the pressure is increased to 22.12 MPa, and at a temperature of 374ºC, water does not boil but is directly converted into steam. This is called the critical point, and the pressure above this critical point is called supercritical pressure. Supercritical pressure with a temperature equal to or more than 593ºC is called ultra-supercritical pressure.
Offline
Like button can go here
#127 2021-12-12 15:41:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Without ground proof of how hard the soils are during the day to night cycle we may need to give a soil preheat coil loop to this monster contraption to make sure that we do not break the digging section due to the ground being so hard.
I would think that its needs to be a meter by 2 meters to fit in from on an arm that could be lowered and raised as needed so long as we do not need it. The flow of maybe liquid co2 or glycol or other working fluids might be what we would want in the coil of tubing which is mounted to a heat reflector set to send it to the ground. I will probably need a bypass value as well for when we do not need it and heat storage tank.
Offline
Like button can go here
#128 2021-12-20 08:47:41
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
On Mars, we must inject large amounts of heat into the ground to melt permafrost. To do this, we need at least 500KJ of heat per kg water, to first heat the ice from -60°C to 0°C, melt the ice and warm the water to ~10°C. I say 'at least' because the ice will be mixed with other solids that will absorb heat and some heat will escape into surrounding materials. We must then desalinate the liberated water. So we are probably looking at around 1MJ of energy per kg of water, most of which is in the form of heat. That works out to be nearly 300kWh of energy per m3 of water - which is around 50x more energy than is needed for desalination of water on Earth.
How does these target values match up with the tray baking to liberate water to a 5 minute duration at 500'C?
Unlike earth the salts are an advantage for electrolysis as we are not intending to drink it and we would be using a sabatier to make drinkable water.
Offline
Like button can go here
#129 2021-12-20 09:22:36
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Mars Water regolith soils 1 foot depth only
Baking soil to 500°C will consume a lot more energy per unit water, as most of the mass heated is not water. But why do we need to do that anyway? You could get most of the water out by heating to 30°C at typical Martian pressures.
I don't honestly know if you can put untreated salty water into an electrolysis cell. Most electrolysis is carried out in alkaline water. Could the membranes, anode and cathode be damaged by lack of chemistry control? Not sure. If you are evaporating the water from the dirt it isn't an issue anyhow as your water is distilled.
What I was talking about in the other thread is bulk water supply for an eventual base. Water for propellants production, industrial uses, agriculture and hotel use. For those sorts of loads, we are going to need tonnes of water per capita. We can only meet those sorts of requirements through bulk mining.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#130 2021-12-21 21:12:38
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
So testing of the bake cycle release of moisture as steam will require sensors to automate the process rather than a strict time for a given temperature. So tray contents will be IR sensor evaluated while in the cycle to watch for temperature changes.
I am thinking that each unit will be outfitted with Mars weather reporting equipment similar to that which is being used on the rovers which has been found to be very robust to limit development.
We will monitor mars https://en.wikipedia.org/wiki/Climate_of_Mars for dust storms and more to ensure success.
We will most likely want a mast camera and possible ground radar to watch the diggers path for large rocks that would cause damage to the unit.
We will also want a neutron detector unit to give measurement of water content that the path contains before gathering to place into the chamber for baking as a means to measure expectations.
The unit will also carry communications equipment not only RF radio but networking computer interface.
The frequency of transmission plays a major role in the quantity of data that can be transmitted per unit time and an IR laser's frequency is far above radio frequency ranges. There are various other ways to pack more data into a carrier signal per unit time, but increasing the frequency is generally how we do it when using radios. That's why 5G cellular communications operate at higher frequencies than 4G, for example.
Radio frequency communications are generally either focused / directional or unfocused / omni-directional. The focused signals can transmit more data per unit time because more of the electromagnetic radiation arrives at the receiving end than with omni-directional antennas, improving the signal-to-noise ratio (strength of the signal) and lowering the transmitter power requirements, but that also requires precise alignment of the transmitter with the receiver over extended ranges, essentially a line-of-sight system as a result, just like lasers.
Offline
Like button can go here
#131 2022-01-23 17:49:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
https://www.greenenergytimes.org/2021/1 … l-warming/
Ice can exist at 0°C (32°F), and if no energy is going into it, it does not melt. Water can exist at the same temperature, and if no energy is being extracted, it does not freeze. There seems to be some sort of balancing act going on here. How does that work?
A calorie (as used in physical sciences) is the amount of energy required to raise one gram of water by 1°C. The amount of energy it takes to melt a gram of ice at 0°C into water at 0°C, without changing the temperature, turns out to be about 80 calories. If you measure the amount of heat it takes to convert a given volume of ice into water, without raising the temperature, and then apply the same amount of heat to the water, you will raise its temperature to about 80°C (176°F). It takes a lot of heat to melt ice.
So to liberate the ice water from the soil which is estimated as being 9kg for a meter square at 1/2 meter depth is why the temperature must be brought up fast to bake it out of the soil. As most of the heat is not used to get the water but to raise the temperature of the soil to allow for the small amount of ice to vaporize.
Offline
Like button can go here
#132 2022-01-28 20:23:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
This post is to thank Void for another outstanding discovery!
http://newmars.com/forums/viewtopic.php … 16#p190616
The post at the link above contains a link to, and snippet from, an article about the comparative efficiency of use of microwaves to soften soil containing frozen water. While the method may not be productive if water is not present, the results indicate effectiveness up to 140 times greater than conductive heating.
It seems to me this research shows a way forward for numerous proposals for Mars settlement activity involving water.
A heat engine (eg a nuclear fission reactor) can produce electricity to generate microwaves, while dumping waste heat into regolith to maintain it's own stable operation. While the dumping process is inefficient (compared to microwave heating) the energy released ** should ** be 100% captured by humans using the process on Mars.
For SpaceNut re Top Layer of Regolith Water Harvest equipment .... Please continue development of your important (to me for sure) work on the first meter problem. You left off with a plan to use scrapers and scoops to collect regolith so that water mechanically bound to the materials could be released. It may turn out that use of microwaves to soften the soil before scooping would permit greater productivity for a given amount of fission reactor energy in your system.
(th)
Offline
Like button can go here
#133 2022-01-28 20:24:57
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
The microwave idea is a good way to thaw frozen regolith. As for harvesting the water content of the frozen regolith, there is a maximum of 10 kg (10 L) of water harvestable for every single percent of water content in a metric ton of regolith. And just what is that water content, 3-4%? 30-40 L max per ton processed? You gotta do a lot more to that ton than just scoop it up.
And that assumes all your harvesting processes are 100% efficient (which they will NOT be). Tons to move and process for only liters of water does not sound very promising to me as a viable resource. I guess it's better than no resource at all, but I think drilling into buried glaciers would yield orders of magnitude more water for a whole lot less effort. Steam down the pipe, water comes back up. Coaxially-nested pipes make this a continuous process.
GW
Offline
Like button can go here
#134 2022-01-28 20:43:22
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
I had been thinking about how hard the ground might be and until its been tested as a prototype in a ground level condition simulating mars we might be over designing for the what if but if we find that its needed the levels required is more than 6Kw of microwave energy to penetrate into the ground that might be frozen. We are just trying to defrost it but not release the water from it.
Microwave Thawing of Frozen Soil abstract
On earth they place a blanket over the area which is something for mars which seems not possible. Another is to place a hot box heated by propane not likely as well.
https://www.thawground.com/
Offline
Like button can go here
#135 2022-03-05 20:00:29
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Test of the baking was already done we just need it on a larger scale
Where is the water on Mars?
The regolith in these samples were heated in an oven to 500C°. This caused water, initially frozen inside of the regolith, to be ejected in its gaseous state. This caused the weight of the regolith to decrease by 1%. Thus, when the amount of water contained in these samples was measured, the regolith must have been 1% water and 99% other stuff. But the regolith measured in these samples was exceptionally dry and lost a lot of water content during the time interval when the regolith was extracted and stored before being cooked to 500C°. We think that the average regolith on Mars is more like 3% water.
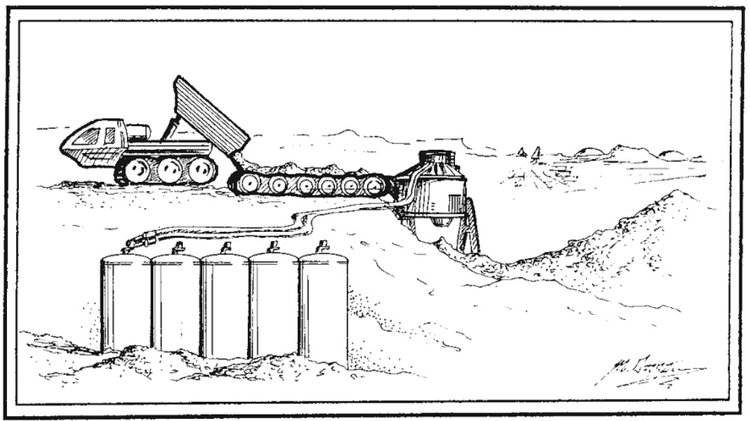
One technique used by that group of Martian pioneers to extract water from the regolith would likely be the following: a dump truck would dump regolith onto a conveyor belt (see illustration above); the conveyor belt then transports the regolith to an oven powered by a 100KWe (kilowat-electric) reactor. The oven would cook the regolith to 500C°, hot enough to release water from that regolith in its gaseous state. The water vapor would then be collected in a condenser and subsequently stored inside of large containers. But not all of the power generated by the reactor would get converted into electricity to power the oven; the overwhelming majority of this power would get converted into waste heat. This waste heat could be used to cook additional regolith and used to extract even more water. Such a system could extract 14,000 kg/day of liquid water.
Offline
Like button can go here
#136 2022-03-12 14:43:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
“The project, supported through ESA’s Technology Development Element, will include the initial design of a large scale reactor device to periodically extract oxygen from soil, what we term ‘oxygen farming’. Solar UV irradiation will then replenish their oxygen supply within a matter of hours. The estimate is that a 1.2 hectare (3 acre) area would yield enough oxygen to keep a single astronaut alive.”
https://www.esa.int/Enabling_Support/Sp … en_farming
Viking’s ‘Labeled Release’ experiment applied micro-nutrient liquid to a Martian soil sample, which released copious amounts of oxygen in response. Some authorities interpreted this result as evidence of microbial life on Mars – except that even after the sample was sterilised with 160°C heat this oxygen production continued. Meanwhile other Viking experiments found no traces of organic chemicals.
https://www.esa.int/ESA_Multimedia/Imag … or_concept
so we now have a bonus with the heating but I need to find something that indicates how much is released.
Offline
Like button can go here
#137 2022-03-12 18:30:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Perchlorate Radiolysis on Mars and the Origin of Martian Soil Reactivity
If mars has this much rock at the sites to which we are looking to turn into water we may need to alter the digging method

Offline
Like button can go here
#138 2022-03-18 18:22:12
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
I am in awe with regards to the quantity of fuel required for an every other mission cycle for the Large ships dead head mass of 5000 mT.
Its not going to be done with soil water for sure even with a 52 month to get it done in. In fact that is also quite a bit of Co2 as well.
The issue then for a starship support is how much cargo can we land to build up capability for fuel creation but can we live on mars for that long?
Offline
Like button can go here
#139 2022-07-12 17:35:01
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
This repost makes meantion of teh larger Krusty unit that I referenced earlier in the topic to make use of more heat and energy to power the unit to dig regolith to bake out water and to gather co2 to make starship sized quantities of fuel.
MIT design for Mars propellant production trucks wins NASA competition
https://www.marsdaily.com/reports/MIT_d … n_999.htmlUsing the latest technologies currently available, it takes over 25,000 tons of rocket hardware and propellant to land 50 tons of anything on the planet Mars. So, for NASA's first crewed mission to Mars, it will be critical to learn how to harvest the red planet's local resources in order to "live off the land" sustainably.
On June 24, NASA announced that an MIT team received first place in the annual Revolutionary Aerospace Systems Concepts - Academic Linkage (RASC-AL) competition for their in-situ resource utilization (ISRU) design that produces propellant on Mars from local resources instead of bringing it from Earth.
Their project "Bipropellant All-in-one In-situ Resource Utilization Truck and Mobile Autonomous Reactor Generating Electricity" (BART and MARGE) describes a system where pairs of BART and MARGE travel around Mars in tandem; BART handles all aspects of production, storage, and distribution of propellant, while MARGE provides power for the operation. After presenting their concept to a panel of NASA experts and aerospace industry leaders at the RASC-AL Forum in June, the team took first place overall at the competition and was also recognized as "Best in Theme."
"Previous ISRU concepts utilized several different small rovers and a fixed central plant, but MIT's BART and MARGE concept is composed of essentially just two types of fully mobile, integrated large trucks with no central plant," says Chloe Gentgen, PhD candidate in the Department of Aeronautics and Astronautics (AeroAstro) who served as team lead for the project. "The absence of a central plant enables easy scalability of the architecture, and being fully mobile and integrated, our system has the flexibility to produce propellant wherever the best ice reserves can be found and then deliver it wherever it is needed."
Gentgen led an interdisciplinary group of undergraduate and graduate students from MIT, including Guillem Casadesus Vila, a visiting undergraduate student in AeroAstro from the Centre de Formacio Interdisciplinaria Superior at the Universitat Politecnica de Catalunya; Madelyn Hoying, a PhD candidate in the Medical Engineering and Medical Physics program within the Harvard-MIT Program in Health Sciences and Technology; AeroAstro alum Jayaprakash Kambhampaty '22, rising MIT senior Mindy Long of the Department of Electrical Engineering and Computer Science (EECS); rising sophomore Laasya Nagareddy of the Department of Mathematics; rising junior John Posada of AeroAstro; and rising sophomore Marina Ten Have of EECS.
The team was formed last September when interested students joined the project. AeroAstro PhD candidate George Lordos, who founded the MIT Space Resources Workshop and who has led or advised all MIT NASA competition teams since 2017, was a mentor for the project team. Jeffrey Hoffman, professor of the practice in AeroAstro; and Olivier de Weck, Apollo Program Professor and professor of astronautics and engineering systems in AeroAstro, served as faculty advisors.
"One year ago, the MOXIE experiment led by Dr. Michael Hecht and our team's advisor, Professor Jeffrey Hoffman, produced the first oxygen on Mars. Today, we are on the cusp of orbital test flights that will bring us closer to the first human mission to Mars," says Lordos.
"As humans venture to other worlds, finding and utilizing local water and carbon resources will be indispensable for sustainable exploration of the solar system, so the objective of our MIT team's concept is an exciting and topical technology."
The MIT team addressed the RASC-AL theme "Mars Water-based ISRU Architecture," which required delivering the target 50 tons of propellant at the end of each year and the ability to operate for at least five years without human maintenance. A few other constraints were placed, chief among them that teams could rely on one or more landings of 45 tons of mass and 300 cubic meters of volume on Mars, leaving it to university teams to propose an architecture, budget, and a flight schedule to support their mission.
They developed a comprehensive Mars mission architecture and defined a comprehensive concept of operations, from a precursor ice scouting and technology demonstration mission in 2031 to the main propellant production, storage, and delivery mission in 2036. BART is an end-to-end "ice-to-propellant" system that gathers water from Martian subsurface ice and extracts carbon dioxide from the red planet's atmosphere to synthesize liquid methane and liquid oxygen bipropellant. These are then stored onboard at cryogenic temperatures until delivery directly into a rocket's propellant tanks.
BART is accompanied by MARGE, a 40 kilowatt electric mobile nuclear reactor based on NASA's Kilopower Reactor Using Stirling Technology project (KRUSTY, which also inspired the MIT team's name) that generates power from nuclear fission to support long-duration operations on distant planets.
For the team's proposed mission, four tandems of BART and MARGEs will roam the region known as Arcadia Planitia at the mid-northern latitudes of Mars following a prospecting rover named LISA (Locating Ice Scouting Assistant) in search of accessible ice to use for propellant production. The entire system has 100 tons of storage capacity and can produce 156 tons per year, against a demand of 50 tons per year, and requires only three landings.
This also competes with our version of the Canadian drill of which GW is about to update.
Offline
Like button can go here
#140 2022-10-21 17:46:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Climeworks became the first company to suck carbon dioxide out of the air and sell it as a product back in 2017. That’s when its direct air capture (DAC) plant called Capricorn opened in Hinwil, Switzerland. Climeworks announced that it has “complete[d] the commercial operation phase of its DAC facility in Hinwil, leading to the phase-out of its first generation technology
The company's focus has shifted from this size to a much larger unit and into storing co2 deep in the ground.
Offline
Like button can go here
#141 2023-11-08 19:26:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
I believe this work as well for the first foot of material to process.
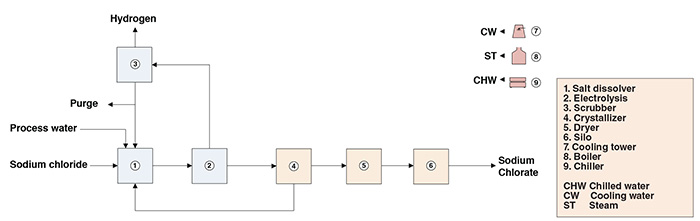
Here is the electro-chemical process for making Sodium Chlorate (NaClO3), using salt (NaCl) and water (H2O) as feedstock:
If you can obtain CO2 and H2O from Mars, then Sodium Chlorate is a storable solid white crystalline compound (much like NaCl), requiring no pressurization or temperature control. This infinitely reusable chemical will split H2 from H2O. Further heating the Sodium Chlorate (NaClO3) to 300C, after the water splitting, will then release the Oxygen.
Sodium Chlorate is used for everything from paper bleaching to cosmetics to pesticides, but the paper bleaching industry is a major user of this industrial chemical product.
That said, Hysata makes a 95%+ efficient reverse fuel cell that splits H2O. This is almost certainly the way to go. H2 from the reverse PEM fuel cell is fed into the Sabatier reactor. The Sabatier reactor combines CO2 and H2 gas into 1CH4 and 2H2O. Alternatively, CO and H2O combine into 1CH4 and 1H2O. Both types of electro-chemical reactions feed into the Sabatier process.
The primary advantage of Glucose is storability. Both Sodium Chlorate and Glucose will store indefinitely with zero pressure, irrespective of temperature (within the limits of Mars surface conditions), because they are solids.
Also the fuel cell
We only briefly touched on an exergy analysis of propellant production, but this is absolutely critical to the success of any propellant production facility, because it dictates the cost of the equipment involved.
Hysata's capillary action reverse PEM (Proton Exchange Membrane) fuel cell (RPEMFC), requires 41.5kWh of electricity to make 1kg of H2 from H2O. H2 stores 39.4kWh per 1kg of H2 gas, when completely reacted with O2 in a 100% efficient combustion or chemical reaction process.
Every 1,000kg / 1t of H2 fuel or feedstock (for making CH4), thus requires 41.5MWh of electrical power input. The H2 content of the Starship upper stage's load of LCH4, is a bit less than 66,000kg, so 2,739MWh / 2.739GWh of electrical power is required just to create the Hydrogen. To produce this much H2 over 1 year / 8,760hrs, requires a constant power output of 312,671 Watts. This would require a massively over-sized solar array to produce 7,504,110Wh of power during just 6 hours of peak sunlight, or a Megawatt-class nuclear reactor.
On its best day, NASA's InSight lander was about to produce 4,600Wh of power from 4.2m^2 solar array. If the panels were kept clean, then 1,632 similar arrays, covering 6,854.4m^2, would be required to produce 7.5MWh of electrical power per day. An American football field covers 5,350m^2. It would probably be safe to assume that at least 2 such arrays would be required to provide the rest of the power for fuel compression and liquefaction. NASA pays about $1M/kW for these very advanced triple-junction solar arrays, so the purchase cost for 2 arrays, not the cost to ship them to Mars, is about $15B USD. For comparison purposes, the recently built 1.25GWe Watts-Bar #2 commercial nuclear reactor costs $4.7B USD to construct. This is the true cost of solar power on Mars- the electrical power to split enough Hydrogen to refuel 1 Starship would cost more than an Earth-based nuclear reactor producing 133X to 167X more power. That is why NASA has restarted its nuclear power programs. Solar power was always going to be a severe limitation. Politics killed the space nuclear programs back in the 1970s.
That is where our energy requirements start. The resultant fuel and oxidizer must then be compressed and cooled using a cryocooler.
Back in the real world, no process is ever 100% efficient. PEMFCs (Hysata's RPEMFC consumes electricity to make H2, whereas a forward or "plain old" PEMFC consumes H2 and O2 to make electricity) are typically 70% efficient. That means 27.58kWh of electricity comes out the other end of a normal PEMFC. Maybe Hysata's fuel cell could be run in reverse to produce electricity, but I doubt it. The device was deliberately engineered to be very efficient at producing H2 gas, whereas fuel cells that produce electricity are typically used in mobile applications where size / weight / power density is what matters most. Efficiency takes a backseat to raw performance. Some types of fuel cells are more efficient, some less, but PEMFC efficiency is a well-known / well-understood / highly mature technology, in use for more than 50 years. Since PEMFC operates below 100C, more common / lighter weight / lower cost materials (plastic and Aluminum) are suitable. Furthermore, the generated steam can cool them in a type of closed loop cycle. As a result, the engineering and service life of such fuel cells is something that can be accurately estimated and a wealth of knowledge available for designing newer / better / more efficient models exists. Solid Oxide fuel cells (SOFCs), which run directly on CH4 without reforming the gas into H2, are very high temperature and made from very exotic refractory metal and ceramic materials. The advantage of SOFCs is that they can readily achieve up to 85% efficiency, with greater power density than PEMFCs. The downside is that they are so hot that they can crack and they are difficult to hold onto and insulate, being as hot or hotter than the hot section of a jet engine. Heating them up or cooling them down too quickly will result in cracking and potential catastrophic failure which includes an O2/CH4 explosion.
Rocket engines, especially H2-fueled engines, typically run fuel rich to avoid melting the engine. Pure O2/H2 flame temperature is about 2,727C, which is hot enough to melt all metals commonly used in rocket engines, like the proverbial blow-torch taken to a tub of butter. Excess H2 fuel has a fantastic ability to absorb excess heat, so the extra fuel in the exhaust knocks the flame temperature back to something that is tolerable by a regeneratively cooled engine, which uses some of the H2 fuel to absorb heat from the rocket chamber / threat / nozzle before being fed into the combustion chamber or dumped overboard.
Similarly, all piston and gas turbine combustion engines also require considerable cooling to avoid melting. A very healthy portion of the generated heat is actually heating up the engine, which must be dissipated into the surrounding environment, rather than producing mechanical work output of some kind. The LH2-fueled piston inline 6-cylinder race car engine that provides electrical power and pressurization to the LH-2 fueled Centaur upper stage of the Atlas-V / Delta-IV / Vulcan families of orbital launch vehicles, powered by LH2-fueled RL-10 engine, has to be massively de-rated from 600hp+ to less than 30hp, again, to avoid melting the engine, which is cooled by H2 boil-off gas.
Either way, quite a bit of heat energy is inevitably lost. This places the overall efficiency of any process producing fuel and oxidizer, with severe mass and energy input constraints imposed by the need to ship all equipment to the surface of Mars, at an absolute premium. Efficiency is the name of the game.
Offline
Like button can go here
#142 2023-11-10 11:11:01
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
tahanson43206 wrote:kbd512 wrote:Here is the electro-chemical process for making Sodium Chlorate (NaClO3), using salt (NaCl) and water (H2O) as feedstock:
https://www.chemengonline.com/wp-conten … um-21b.jpg
If you can obtain CO2 and H2O from Mars, then Sodium Chlorate is a storable solid white crystalline compound (much like NaCl), requiring no pressurization or temperature control. This infinitely reusable chemical will split H2 from H2O. Further heating the Sodium Chlorate (NaClO3) to 300C, after the water splitting, will then release the Oxygen.
Sodium Chlorate is used for everything from paper bleaching to cosmetics to pesticides, but the paper bleaching industry is a major user of this industrial chemical product.
That said, Hysata makes a 95%+ efficient reverse fuel cell that splits H2O. This is almost certainly the way to go. H2 from the reverse PEM fuel cell is fed into the Sabatier reactor. The Sabatier reactor combines CO2 and H2 gas into 1CH4 and 2H2O. Alternatively, CO and H2O combine into 1CH4 and 1H2O. Both types of electro-chemical reactions feed into the Sabatier process.
The primary advantage of Glucose is storability. Both Sodium Chlorate and Glucose will store indefinitely with zero pressure, irrespective of temperature (within the limits of Mars surface conditions), because they are solids.
I missed this before. Assuming I have understood correctly: the reaction between sodium perchlorate and water, removes the oxygen atom from the water liberating hydrogen. The perchlorate can then be regenerated by heating to 300°C. The net reaction:
2H2O + heat @300°C = 2H2 + O2
The really interesting thing about this reaction is that heat at this temperature range is produced in abundance by light water reactors.
There is no advantage whatever to using glucose as a propellant if we are starting with CO2 and H2 as syngas. Methanol has higher energy density, is a room temperature liquid and is easier to synthesise.
CO2 + 2H2 = CH3OH. I cannot remember which catalyst is needed. But once you have methanol, reactions over a zeolite catalyst can build up longer chain hydrocarbons. If we can synthesise methanol we have the feedstock for plastics industry. And of course there is dimethyl-ether:
2CH3OH = CH3-O-CH3 + H20
This fuel has a boiling point of -24°C and has 80% of the volumetric energy density of diesel. As the fuel is already partially oxidated, the energy density of DME/O2 bipropellant is comparable to LOX/RP1.
Methanol doesn't freeze until -97°C. It is also non-corrosive to steel. So you can store this on Mars as a liquid in lightly pressurised (<1 bar) steel tanks on the surface. It may be the propellant of choice for long-range surface vehicles. Methanol thermophysical properties:
https://www.engineeringtoolbox.com/meth … _1209.htmlAt -50°C, methanol remains liquid and vapour pressure is 10mbar. So this fuel is storable at ambient pressure on Mars. It can be stored in thin walled steel tanks. The vehicle fuel tank will not need pressurisation either and can work much the same as an Earth gasoline tank.
Offline
Like button can go here
#143 2023-11-10 11:30:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Seems this is one of the best way forward if no solid ice fields are found near a landing sight.
I would suggest a smaller version with just a single starship as the trail to make fuel and to give data of landing sights stability to land other ships near it.
My previous assumption was incorrect. Sodium chlorate is produced by electrolysis of a sodium chloride water solution.
https://link.springer.com/article/10.10 … 017-1100-3NaCl +3H2O = NaClO3 + 1.5H2.
The process requires some 5000kWh of electric energy per tonne of perchlorate. Some 60kg of associated hydrogen are produced, which equates to 300MJ/kg of H2. So the perchlorate process would be less efficient than ordinary water electrolysis.
*******************Additional (1): The copper-chloride process would appear to be the most promissing thermochemical process.
https://en.m.wikipedia.org/wiki/Copper% … rine_cycleThe highest reaction temperature needed is 530°C. This makes the process compatible with a number of Gen IV concepts. Perhaps most promissing is the sodium cooled fast reactor, which operates at temperatures of 550°C. A 530°C operating temperature allows for some thermal gradient across heat exchangers.
Additional (2): Direct thermolysis of water is potentially interesting. Go to slide 15 in the presentation below.
https://www.slideserve.com/jontae/lectu … productionAt 1730°C, some 0.69% of water molecules dissociate into hydrogen. I find myself wondering if it is possible to build a high temperature nuclear reactor that directly dissociates water into H2 and O2 within the core itself. The fuel would be uranium oxide, clad in tungsten, with a solid metal oxide sleeve to protect the clad from oxidation. Hydrogen would be extracted by passing the superheated steam across metal oxide tubes. A pressure gradient between the outside and inside of the tubes would allow hydrogen to diffuse through the grain boundaries into the interior of the tube, whilst the O2 and H2O molecules are too large to diffuse in this way. The remaining mixture of steam and O2 would exit the reactor through a counterflow heat exchanger, through which additional steam is added to the reactor.
In this way, nuclear heat can be converted directly into H2 and O2 within the reactor. This is probably something we could build on Mars, away from the stifling control of organizations like the NRC and ONR.
Offline
Like button can go here
#144 2023-11-18 16:53:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Not to take the other topic off creation of methane from carbon and water source.
Most likely the tanks of a ship will be made use of rather than tying up mass with bringing others for this purpose. We are still going to need to prototype this unit for testing so that its capability is well known for its robustness and output as well as energy consumption.
I would use a ships tank as a holding location for the gaseous un-liquified methane and for the oxygen from the electrolysis of the water before liquifying them for later use as these are capable of that as the liquid test was at 8 atmospheres. The rate that the starship will use it at is based on the Starship’s Raptor engines burn about 3.55 kilograms of LOx for every 1 kilogram of LCH4 that gives the total 1200 mT of fuel that is required. Thats going to make methane roughly 260 mT with 940 mT oxygen.
Until its ready this is what we will need for a tank farm. At the Texas launch site appear to store about 1000 cubic meters (~35,000 ft^3) of LCH4, while the vertical tanks would have stored about 1800 m^3. The vehicle's payload bay, measuring 17 m (56 ft) tall by 8 m (26 ft) in diameter, is the largest of any active or planned launch vehicle; its internal volume of 1,000 m3 (35,000 cu ft). only has this much room for the water and Carbon to be shipped from earth that totals 100 mT payload capability from Earth.
CO2 is 12 + 2(16) = 44 grams
4H2 is 4(2) = 8 grams
CH4 is 12 + 4 = 16 grams
2(H2O) is 2( 2 + 16) = 36 grams
combustion of 2 CH4 + 3 O2 ———> 2 CO2 + 4 H2O
3o2 is 3(2(16)) = 96 grams
2(CH4) is 2(12 + 4) = 32 grams
2 (co) is 2(12+8) = 40 grams
4 (H2O) is 4 ( 2+16) = 72 grams
That is 854.5 m3 the density of water is 1,000 kg/m³ or 854.5 mT. is all the cargo a starship can hold for water.
To create the 240 mT of Methane we need
Carbon to make 240 mT of methane appears to be 180 mT.
we need just 60 mT Hydrogen from the water.
H= 4 x 2 = 8
O= 4 x 16 = 64
4 H20 = 72
oxygen which is required 940 mT required to burn the methane.
oxygen from 1 time full cargo of 854.5 mT of the water is 759.55 mT
Hydrogen is 94.95 mT
So far this is not a 1 time shipment and will require more starships to even do this as the oxygen is falling short.
Offline
Like button can go here
#145 2024-02-12 19:04:46
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Not sure if going in a circle like an open pit mine would change quantity of water yield but it serves to create living space.

Offline
Like button can go here
#146 2025-09-22 17:31:52
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
https://www.marspapers.org/paper/Gurrea_2021.pdf
Something that I had calculated a long time back was the quantity of ships needed to support 1 crewed for return to earth.
Pioneer Astronautics demonstrated a reactor capable of producing 1 Kg a day of methalox fuel from hydrogen and carbon dioxide while consuming a power of 700W. For 710 tons in 400 days that is 1.89 MW. (Zubrin et al., 2013) Assuming 400 days to produce the 710 tons of fuel needed, 352 tons of water (for electrolysis) and 1.89 MW of power would be needed. Using the methods and assumptions detailed in section 4.3 (including a 20% margin for safety), the solar infrastructure would be:
• 229.2 tons in mass.
• 3437.4 cubic meters in volume.
• 57290.1 square meters in area.
The deployment would require 5 to 6 Starships (volume constrained) and significant deployment operations and maintenance. Power remains one of the most significant challenges of a Mars mission architecture that accounts for the return of the astronauts. As with issue 1, failure in this area would result in loss of crew.
Offline
Like button can go here
#147 2025-09-23 15:16:44
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
Now how to shrink the system so that not as many ships are required?
Offline
Like button can go here
#148 2025-09-24 06:09:53
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re #147
Supplies do not need to be shipped inside vessels that are designed for atmosphere.
On Earth, shipments of various kinds are often shipped by barge. A space "barge" would be open to vacuum. Supplies would be packaged on Earth for shipment in open vacuum.
GW Johnson has described a family of space tugs for all sorts of applications from launching human ships to Mars and receiving them on return, to just about everything in between.
The idea of shipping everything inside an atmosphere protected shell is understandable because that is all that humans could achieve up to this point. However, there are opportunities for business ventures that save cost by developing and marketing deep space shipping structures that are designed for the purpose, including "barges" and space tugs, and all the infrastructure that would support them.
(th)
Offline
Like button can go here
#149 2025-09-24 14:08:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Mars Water regolith soils 1 foot depth only
cargo requires both on first missions to go with a crew but for preloading one can use the slow barge method it still needs both depending on the item being shipped.
current goods go by Dragon truck which has both for all sorts of items including food.
to which this is not about cargo but building material for insitu refueling.
edit
something to remember is that a space tug goes orbit to orbit but does not land on a planet.
They would require an orbital platform to transfer the cargo to a down freighter to the planets surface.
End result of processed and dried regolith means it is ready for brick making if binders are present to build with plus a kiln.
This starts all insitu processes and uses.
Offline
Like button can go here