New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#76 2021-10-20 19:20:11
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
We do not need to create and save 2400 tons of propellant in 365 Earth days, or 354 Sols as its only 1200 mT for 1 starship crew to return with and the time is longer.

http://clowder.net/hop/railroad/EMa.htm
case of Mars, the period is 780 days (2.1 years)
Modified table contents
Leave Month Day Year Arrive Month Day Year travel period Days
2022.6033 8 7 2022 2023.3120 4 22 2023 0.7087 258.6755
2024.7387 9 26 2024 2025.4474 6 11 2025 0.7087
2026.8741 11 15 2026 2027.5828 7 30 2027
2029.0095 1 3 2029 2029.7182 9 19 2029
Decision to launch time to make fuel is the difference of when you arrived to when you can launch next
2024.7387-2023.3120=1.4267 x 365 = 520.7455 days
2026.8741-2025.4474=1.4267
2029.0095-2027.5828=1.4267
Keep in mind that this is a circular timing the mars path with make the times get longer currently
Found this after creating the above content
The 2 landed cargo ships only need to be half filled each to get the total fuel that you need and they need to be modified with cryo generators to reduce the boil-off as the ships set idle waiting for nearly 500 days after it lands to be able to fill the crewed ship just before the return trip occurs.
The near zero boiloff off unit needs power and tanks to make use of plus some valves and plumbing to make the system work. First vent any buildup of pressure from boiloff into the header tanks which have the altered path to a cryo-generator for both the oxygen and methane and return the fuel back to the lower tank that are being filled.
The inlet from the insitu processing is sent from the ground to the header tank where the cryo-gen units does its thing and send it on its way to the main tanks.
So the only question is what does a starship have for a surface power system on a cargo ship beyond the wing control batteries?
https://marspedia.org/Mars_mission_duration
Keep in mind we will load these ships for both missions heavy for a long transit time so the duration will from when the cargo land to when the crew launch will be shorter.... post 44 mission profile
Now going back to the number go to post 10 which gives a baseline system that starts with sources of ice with no equipment mass and solar panels with no batteries, the electrolysis unit operates just 10 hrs out of the day, of which its 2 years to get 300mT of fuel ( 60mT methane + 240mT oxygen), at a mass of 80mT for the total design.
We need 2 ea of the complete 80mT packed units per starship which exceeds the mass of a starsip cargo ship capacity in slow transit mode which is stated as 150 mT.
That is why I stopped the calculations until the bottle necks of mass, power and other items can be solved and or efficiency of each can be created or found.
Offline
Like button can go here
#77 2021-10-20 19:52:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
You are describing (as nearly as I can tell) a batch process:
1) Scrape regolith and deliver it to a hopper
2) Intake regolith from hopper and pack it into an oven for baking
3) Bake regolith until (a) all free volatiles are expelled and (b) all chemically bound volatiles are expelled
4) Simultaneously (in parallel) collect CO2 from the atmosphere
5) From step 4, process the collected CO2 to yield CO and O2
6) From step 3, collect water for processing, and save other valuable volatiles (*)
7) Simultaneously, make methane, compress and cool it, and save it in extended storage container(*) The baking process ** should ** yield bricks which can be transferred back to the regolith work area to make a landing pad
Not quite
The digging unit and the hopper needs to move with the baking unit to reduce un-needed motion that wastes time for the digging unit and we do not want to add more equipment to do the moving which has some level of tankage.
We also do not want to pack the tray with soil from the hopper just leave it loose to accelerate out gassing of water ect.
we do not use any co for anything nor do we break the co2 down for use. 2Co with 2H2 would be the RGWS (reverse gas water system) to generate water. That's a process for when the crew would arrive as part of the air scrubbing recycling units.
The electrolysis unit and methane creation as well as the cryo coolers must be stationary which means the water must be trucked to the unit from the end cycle of the temporary tank on the digger baking unit.
Water truck equipment would move fast such that it does not effect the timing as the digger/ baker keeps going once the truck leave with its load of water to bring it to the electrolysis unit as its quick to pump the water out and to have multiple trucks for that purpose. This is a possible battery unit with recharging on the digger/baker from the sterling generator.
We need lots of digger/bakers units to cover the field grid and at this time can only hope for higher water content to improve yield rates.
Source document indicate we need per day 1.22 mT of co2 and 1.89 mT of water of which we need 4 times plus due to time length goal that amount to do a starship,
6.1 mT of co2 daily is required or 244kg hr
9.45 mT of H2o daily is required or 378kg hr
9kg of water is in a 1m x 1m x o.5m which means we need to gather and process 378 / 9 = 42 m squares gathered each hour
Baking tray is a quantity of 5, at a 10 cm depth that is 1m x 1m for each meter square dug up for processing and we need to spread out 42 of these making the over 42m x 5 m or some shape that we can manage (15m x 15m area) that is even large or multiple tray one over the other. What ever can be proven to work....
So far I have found that we need to get overall mass down, power requirement down and gains on throughput up to achieve the goal in the payload mass envelope of a starship in slow transit.
Offline
Like button can go here
#78 2021-10-20 21:30:16
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re topic ....
There seems to some drift going on here ...
I went back in the topic to find this:
For SpaceNut ... GW Johnson has packaged the mission requirements, in the Starship is Go Topic...
From post by GW Johnson:
Here's the rates. It's the same 1200 tons of propellant, regardless of stay time! Water 540 tons/300 days = 1.80 tons (TONS!!!) per day. CO2 660 tons/300 days = 2.20 tons (TONS!!!) per day.Now add life support needs for water and oxygen to that.
From Starship is Go topic
http://newmars.com/forums/viewtopic.php … 02#p186002
***
SearchTerm:Mission requirements for refueling a Starship
SearchTerm:Starship refueling on Mars 1200 tons of propellant
***
My understanding of the topic was that it was seeking to determine the equipment needed to fill one Starship in an Earth year.
GW Johnson gave the figure of 1200 tons to fill a Starship, and that seems to me like a reasonable number to stay with.
At some point, a fixed chemical factory fed by loads of regolith became a small train following a payloader across the terrain.
As our imaginations catch fire, we seem to be adding bells and whistles to what should have been a simple process.
If you had a ** real ** chemical engineer helping you, I'll bet he would be able to give you guidance on how to set up a plant on Mars to make propellant.
(th)
Offline
Like button can go here
#79 2021-10-20 21:48:34
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
Data from post 20 early in the topic and it contains no days at all that I worked out based on equations to fill a starship's 1200 mT of fuel with and what needed to be the inputs from atmosphere and soil water.
We keep having a time frame interjected that is not valid....
water gathered to make methane oxygen with co2 mT from the Atmosphere
540mT 240mT 960mT 660 mt
I have taken a known design and values to solve for the equipment for the soil water and for the co2 even though the design as it stand exceeds the starships capability and what I am trying to get to adds to that growing even larger....Which does not support any follow up mission in a mars launch cycle and greatly exaggerates the number of 4 starships that must land in a given area to get to the mass and power numbers for all of the equipment and processes to have it work.
Crewed Starships will have on the order of 1100 m3 forward space (most of which will be pressurized for human habitation), an 800 m3 liquid oxygen (LOX) tank, and a 600 m3 methane tank.
Offline
Like button can go here
#80 2021-10-20 22:03:23
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re topic ....
What I'm hoping you'll create is a single, concise post that contains all the information a planner would need to fill a Starship using a volume of regolith, as you have described.
We are now up to 79 posts, and it seems to me we are spreading information over posts without consolidating the discoveries.
Returning to basics:
The mission is to accumulate 1200 tons of propellant in one Earth year.
The regolith to feed the process is 1200 meters by 1200 meters by 1/2 meter.
https://learn.openenergymonitor.org/sus … er-process
The Sabatier Reaction was discovered in 1912 by French chemist Paul Sabatier and involves the reaction of hydrogen with carbon dioxide at elevated temperatures (300-400C) and pressures in the presence of a catalyst (e.g: Nickel, ruthenium or alumina) to produce methane and water [1].
The reaction is described by the following exothermic reaction:
CO2 + 4H2 → CH4 + 2H2O ∆H = −165.0 kJ/mol
The CO2 comes from the atmosphere (there may be some recovered from the regolith)
The H2O needed to make Hydrogen comes from water harvested from the regolith.
Useful byproducts of the two harvest processes include gases of various kinds and solids from the regolith.
Energy comes from one or more nuclear reactors.
I brought up CO recently, and you correctly pointed out that CO is not needed for the refueling operation.
However, in this forum, we spent a ** lot ** of time working out the use of CO as a fuel for piston engine machinery such as payloaders to scoop up regolith.
It should be possible for you to create a single concise post that shows everything!
(th)
Offline
Like button can go here
#81 2021-10-21 19:36:50
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
I got looking at the hopper and what it does for the task of making sure the rocks and to hold a quantity of regolith ready for allowing it when a pan is under the chute it auto fills and keeps the clumping to a minimum.
Something like this only larger for the task.
It has an auger to mix / break down the clumps and limits the rocks with the grate grid that might get in to cause it to fault the auger and cause a pan to jam at the entrance to the heating chamber.
The unit needs to hold meters typical but it is going to need a low sensor to keep the the digger filling the hopper with a detection of the high level where it will shut off the digger for a period to allow for it to empty a bit before starting back up.
So the cycle starts and fills sufficient to allow for pans to begin to fill as they are detected under the hopper. As it fills it begins to move towards the oven entrance and as the edge of the pan is detected the chute closes momentarily until the next pane is under it. Taking possibly 5 minutes to fill all trays that are to enter the oven. Which is 5 trays per side of a double width unit.
The cycle from entering to exit of the oven is the critical part to ensure we get all of the water that is present from the tray before it exits. The exiting tray is one tray from the end where it turns and starts to dump its contents out as it goes under the conveyor as it returns. Such that we have 5 trays in, 5 trays under and 3 at each end in transition for the total 16 trays attached to the conveyor.
The dumping and the filling are at the same time so no addition time is required other than the time to fill 5 minutes. Cooking time hopefully can be as low as 5 minutes but could go higher depending on water content of the tray. If its 5 minutes that means we get 6 cycles x 2 for a 10 minutes to get 18 kg of water plus x 6 equals a total processed regolith yielding 108 kg of water / hour.
We need to feed all 4 units with a total 9.45 mT of H2o daily is required or 378kg hr divide 4 ways is 94 kg once we have the co2 which means we are on schedule to deliver the needed water.
If the baking time needs to be increased we start to lose the battle against time unless its also an increase of water content yield.
The water tank on the crawling unit signals that its getting full and the water delivery truck pumps up and pumps the water out of it and brings it to the waiting electrolysis unit inlet tanks. Once empty it pulls away to go get water from the next unit calling for it to be gotten.
Each crawler is encoded for a truck to know which unit its approaching and a central unit commands the truck that is ready to go get the water and places the others into ignore that command.
We should send 6 digger, hopper, oven crawler units divide across the 2 ships. We can enable all to start the process and if we are ahead we are guaranteed that we will succeed even if the water content is not quite as good as we would have wished for. Probably 4 water carrier trucks will be enough but to be safe send 6 as well to keep everything balanced.
Offline
Like button can go here
#82 2021-10-22 06:52:30
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut ...
per Google:
Water on Mars - Wikipedia https://en.wikipedia.org › wiki › Water_on_Mars
On September 26, 2013, NASA scientists reported the Mars Curiosity rover detected abundant chemically-bound water (1.5 to 3 weight percent) in soil samples ...
The purpose of this post is to try to find out if you are "baking" volatile gases out of the regolith, or if you are seeking to liberate the chemically bound water. I am pretty sure there is a significant energy investment difference between the two.
Which are you addressing?
Do you have a professional chemical engineer available to help you with this project?
If you do, that person has not yet appeared in the topic.
I am happy to open a forum membership for anyone you are able to recruit to help.
(th)
Offline
Like button can go here
#83 2021-10-22 18:51:47
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
Samples to detect carbon compounds in Mars rock and soil; the SAM takes in solid samples 0.078 cubic centimeters and ground to particles are smaller than 150 microns in diameter.
59 quartz cups that are small ovens for heating the powdered samples to extract gases
Ovens:
Heat most rock samples to about 1,000 degrees Celsius (about 1,800 degrees Fahrenheit) to extract gases for analysis
https://ssed.gsfc.nasa.gov/sam/curiosity.html
https://en.wikipedia.org/wiki/Sample_Analysis_at_Mars
If we wanted to test the out gassing that would be the instrument suite to make use of to verify what is present but this is not what we are striving to do as the temperature is quite some distance from that of the rovers small let alone sample size which is to test the material of the rock which has been crushed up.
https://en.wikipedia.org/wiki/Volatiles
On planet Earth, the term 'volatiles' often refers to the volatile components of magma. In astrogeology volatiles are investigated in the crust or atmosphere of a planet or moon. Volatiles include nitrogen, carbon dioxide, ammonia, hydrogen, methane, sulfur dioxide and others.
We will get some of these in varying amounts as the planet shows all of the signs of water and current co2 trapped and dissolved into the grains of regolith soils along with others and these are bonus items since we have done the work..
We are not vaporizing and what we are doing have nothing to do with tiny samples...
No need for a chemist as these are all fact from the master chemist on mars, called a rover and orbiting satellites.
What is needed to move forward are prototypes to finish out the specifications builds of a machine of this type and testing. There also needs to be a new co2 gathering design as well based on heat use that Caliban put forth. With the same holding true for a Starship electrolysis and sabatier units to see how we can improve the efficiencies.
Of course other items to be developed fully are transponder communications, a water truck for mars pick up and delivery, new wheels for the heaviest piece of equipment yet to drive on mars.
Offline
Like button can go here
#84 2021-10-22 20:15:26
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut ....
We are not vaporizing and what we are doing have nothing to do with tiny samples...
No need for a chemist as these are all fact from the master chemist on mars, called a rover and orbiting satellites.
Without a PhD level chemist on your team, your output qualifies for publication in the NewMars forum.
I didn't see an answer to my question ...
Are you planning to liberate water that is chemically bound to the regolith?
I see that you are definitely going after volatiles that are accidentally present, but the amount of such material is going to be vanishingly small on Mars.
In all your posts to this point, have you shown how to liberate chemically bound water from the regolith?
If you have, please show a link to a post where that might have occurred.
If you have not yet posted that information, please do so, and please do NOT clutter the post with 1001 one links about everything that might be remotely related ...
All we need is a short, concise statement of whether you are planning to liberate chemically bound materials (a) and (b) how you plan to do that.
(th)
Offline
Like button can go here
#85 2021-10-22 20:23:12
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
We are no point blank are not breaking down the rock.....
We are no where near vaporizing temperatures or duration.
We are not using reduction to get the water, oxygen or hydrogen via pulverized soil...
Baking water from soil is physics and measurement...
Do you need a chemical engineer to melt ICE....
I must need a weather man to know how cold mars is....
Offline
Like button can go here
#86 2021-10-22 21:06:36
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re topic and reminder that you are thinking of hydration.
Since I was not familiar with the distinction between hydration (as defined by chemists) and chemically bound water, I was misunderstanding what you were trying to do.
I went back to the top of the topic, and finally found text that helped me to (at least a bit better) understand where you are coming from.
http://newmars.com/forums/viewtopic.php … 88#p185688
https://www.extremetech.com/extreme/167 … sity-rover
If you heat up a cubic foot of Mars soil, you can harvest around two pints (one liter) of water. According to new data returned by NASA’s Curiosity rover, this isn’t just a one-off lucky find, either: It seems that most of the dirt on Mars harbors large amounts of water.
Quoting from the original article by ExtremeTech.com...
Basically, the GC heats up a soil sample by passing hot (~835C, 1535F) helium gas over it, and then measures the gases that emerge. In this case, by far the most common gas released (between 1.5% and 3% by weight) was water vapor. Per cubic foot of Mars soil, this equates to a lot of water — around two pints, or almost a liter.
The question I've been dealing with is the meaning of "hydration"
The article at the link below addresses the question:
https://www.sciencedirect.com/topics/ea … /hydration
Weathering and Soils Geomorphology
J.C. Dixon, in Treatise on Geomorphology, 20134.14.2.3 Hydration
Hydration results in the addition of water molecules to a mineral structure but without accompanying dissociation occurring in hydrolysis. In short there is no reaction between water and mineral, rather water is simply added to the structure of the mineral. A simple example is the formation of gypsum as a result of the addition of water to anhydrite. In addition, some clay minerals are especially susceptible to hydration as water molecules enter and leave interlayer locations such as montmorillonite (Olson, 2004). Although principally an expansive rather than chemical process hydration does produce secondary minerals such as hydrobiotite which commonly occur in arctic (Isherwood, 1975; Watts, 1981) and alpine (Dixon, 1983; Dixon et al., 1984) soils.
If I understand (now) what you've undertaken, it is NOT to secure water that is chemically bound to the regolith, but instead to shake loose molecules of water that are present in the mineral structures but not chemically bound to them.
From this I deduce that if you proceed as you've been planning, you would send the de-hydrated/dessicated regolith back onto the surface of Mars, where it would (presumably) immediately go back to work collecting stray molecules from the atmosphere.
In other words, if you harvest the volatiles one day, and just let the regolith do it's thing, you can come back another day and harvest more volatiles.
(th)
edit content that should be here:
I understand that the Martian regolith is functioning as a desiccant, then the procedure you are considering is equivalent to the procedure used on Earth to refresh a desiccant.
Once you have "cleaned" the desiccant, you can lay it back down and it will immediately go back to work collecting useful molecules from the atmosphere of Mars. In other words, you would be working with a renewable resource.
Offline
Like button can go here
#87 2021-10-22 21:33:15
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
True that it will absorb more over time and will as well become packed by mars cycles of warming and cooling naturally.
I have been able to prove the content, the area to excavate and the time required as well as the levels of heat to do so. What I need now are near mass estimates for the number of units required...
The mass constraints of 2 cargo starships of 150 mT slow transit to mars is borderline at best if we do not get any mass or energy improvements period even when finding solid ICE which would require a much larger power system and equipment to make it happen from glacier fields.
Finding such a field below the surface would require a different approach to getting it once found.
If we have the margin of mass once we reduce the system then we can look at what to do with additional equipment to take the processed regolith rather than dumping.
The making of bricks require compacting and a binder like sulfur due to the high Iron content that we will find all over mars.
We do have a topic for this and will find and place the link.
wow post from Nov. 2016
The sulfur brick conversation has come up before but I am not able to find the topic that it was in but here is some information on the work status for using sulfur.
PRODUCING A BRICK FROM A MARTIAN SOIL SIMULATE
Materials Scientists Make Martian Concrete
Sulphur Bricks and Super-Arches
content from 2 different posts
Adobe and rammed soil based materials could be very cheap, as we would be mixing Martian fines with water and baking them in a mould. About 90% of the water can be recovered. Embodied energy about 100KJ/kg - 0.2GJ/m3, assuming about 10% water by volume and an equivalent amount of energy for drying the brick, say. For baked (fused) house bricks, say 3MJ/litre, or 3GJ/m3. Of course, a hole in the ground or simple berms of unprocessed regolith could be even cheaper. Stone is relatively energy cheap, but its irregularity may make it labour intensive.
Compressed soil brick: embodied energy = 0.1GJ/m3; typical crush strength = 2-10MPa. Energy per unit strength = 10-50MJ/MN.
Adobe: embodied energy = 0.2GJ/m3; typical crush strength = 2MPa. Energy per unit strength = 100MJ/MN.Martian soil is highly basic and contains a higher concentration of gypsum than would typically be found on Earth. So it should make an effective binding agent.
Martian adobe may well be more efficient than I have calculated here, due to its gypsum content. It would make sense in situations where only low strength is needed and concrete shells would be too thin to be stable. Small structures may work better if Adobe is used.
Offline
Like button can go here
#88 2021-10-23 06:44:31
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re topic ...
Now that I understand that you are thinking of refreshing the desiccant properties of the regolith by baking batches of the material to release any captured volatiles that may be present, I have a better sense of why you are (apparently) thinking of a mobile volatiles collection unit.
It would operate a bit like a combine, taking in regolith on the front end, and discharging desiccant on the back end. Such a machine would need it's own power supply, and a small nuclear reactor seems well suited for the purpose. The problem of dumping heat from the reactor would appear to be addressed by delivering the heat output of the reactor to the regolith being processed.
Surely you need help from a qualified reactor designer to develop the specifications for the reactor.
You have successfully evaded need for a qualified chemist by eliminating chemistry from the process of volatiles collection.
You won't be able to eliminate the need for qualified engineers to help with design of the rest of the system:
1) Intake subsystem
2) Sensors to monitor intake and feed data to control computer
3) Baking subsystem (related intimately to reactor design)
4) Reactor design
5) Vehicle structure
6) Vehicle mobility subsystem
7) Volatiles collection subsystem
8) Desiccant discharge subsystem
9) Control computer with communications to base control computer
You'll surely need help from qualified aerospace engineers to design the delivery mechanism to put your device on the surface of Mars.
There is no need for the CO2 processing system, or the Methane production system to be mobile.
You'll need help from qualified mechanical engineers to help with design of the CO2 collection and processing system.
You'll ultimately need supervision by a qualified chemical engineer to secure funding for your Methane production system.
Your collection of posts in the NewMars forum certainly is gaining potential value over time, but it will (probably) be read by relatively few people beyond the initial reading that almost all posts receive.
If you want to see your hard work published in a wider media setting, you're going to need help.
(th)
Offline
Like button can go here
#89 2021-10-23 08:09:38
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
Thanks for the list to outline a paper to from the posting content.
My resume payload is on the way to you...
I am still needing to look at the quantity of mars sweep air being collected as that impacts the overall co2 picture of the remaining collection system.
I also think that the water electrolysis unit and sabatier reactor can be external from the ship. It needs to be packaged correctly and modified as well as upgraded with more efficient system components. As that design can be improved on just in the cycle of the electrolysis unit that is off during the night in the current design. That along would shrink the power requirement...
Offline
Like button can go here
#90 2021-10-23 09:46:11
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re #39
It would be a nice surprise if you were to decide to convert all this hard work into an actual paper, or better yet, an article in a recognized hardcopy format periodical. A proposal along these lines ought to be of interest to Popular Mechanics or Popular Science, although I'm not sure either is still available.
I see article citations from Popular Mechanics on the Internet feed from time to time, but don't know if it exists in hardcopy forml
It is possible that GW Johnson may be thinking about publishing an article about his Orbital Refueling concept.
***
Something for you to be thinking about (assuming you are serious about this topic) ...
The lander for your equipment does NOT have to look like a Starship.
Once all the equipment is delivered to Earth orbit, it can be packaged in a very different vessel designed to land on Mars and NOT to return.
Therefore, it can be squat and fat and ideal for the base station for manufacture of fuel for ** real ** Starships.
It is possible you might be able to enlist an aerospace engineer to help you design such a lander.
I'd like to see a periodic summary of the progress you've made, as you think through the great variety of challenges your vision has encountered already, and ** will ** encounter in days ahead.
The distribution of knowledge and insights over nearly 90 posts is not helpful to anyone except a dedicated scholar.
One post a week, with bullet points for the challenges and solutions you've found would be helpful.
One 80 character line per challenge would be about right.
(th)
Offline
Like button can go here
#91 2021-11-03 21:12:55
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
So far the mass required for 2 starships would make use of the maximum on orbit leaving values on the slow transit to mars and hopefully it can land safely with the much greater tonnage of close to the 300mT payloads each which can be had.
This would allow for the maximum equipment to make the fuel with.
I believe that the thermal energy from the digging unit can also collect much of the co2 we need as well at the same time.
Offline
Like button can go here
#92 2021-11-20 10:24:32
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only

The most recently discovered Starhsip white paper seems to have an error in the fuel tanks sizing. They indicate that the Crewed Starships will have an 800 m3 liquid oxygen (LOX) tank, and a 600 m3 methane tank.
Offline
Like button can go here
#93 2021-11-22 10:43:35
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
Time to work the Co2 gathering. The turbine and super critical temperature rise to power the turbine and produce the amount of co2 for use seems quite possible with making use of the existing heat from the over wash gasses.
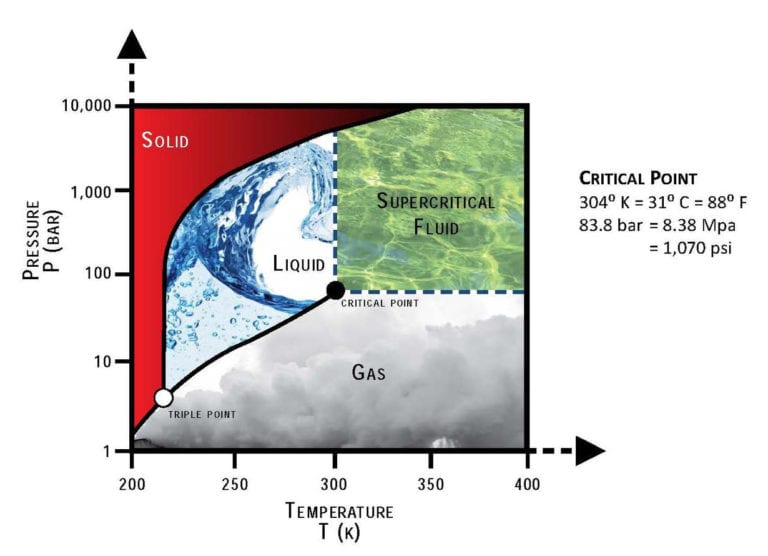
Can Carbon Dioxide Replace Steam to Generate Power?
The term "supercritical" describes the state of carbon dioxide above its critical temperature and pressure, 31 degrees Celsius and 73 atmospheres. Under these conditions, carbon dioxide has a density similar to its liquid state and fills containers the way it would as a gas.
Coffee producers are already using supercritical carbon dioxide to extract caffeine from beans. Materials companies are also using it to make plastics and ceramics.
We do have both water and mars heated atmosphere via the inlet of the regolith into the heating chamber which can be use or in this case reused to power and turn the turbine. The current chamber design has blowers feeding in mars air into the chamber to give a means to draw the water and other gasses into the collection system where its cooled to separate out the water from the remaining.
https://en.wikipedia.org/wiki/Supercrit … on_dioxide
The typical reference is about electrical power creation and what we are interested in is the rotation to make the turbine spin. The creates the needed vacuum to pull more co2 from the Mars air.
https://www.energy.gov/sites/prod/files … urbine.pdf
Innovative power generation systems using supercritical CO2 cycles
Supercritical Carbon Dioxide Gas Turbines For High-Power Generation
Offline
Like button can go here
#94 2021-11-22 10:54:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re Post #93
Thanks for posting your research into this technology ... Not having time (at the moment) to read your numerous links, I'll just applaud your investigation and hope for positive results.
73 atmospheres of pressure ** sounds ** like a lot to me, but it may be quite common in industry.
Energy is required to compress ambient Mars atmosphere to 73 standard atmospheres. I presume you are using a nuclear reactor to provide the needed energy, although that is not mentioned in what I saw. I might well have missed that.
I'll be interested if someone has time to read all that material and provide an estimate of the efficiency of production of electricity from this system. The heat from the reactor is going to be wasted entirely unless some of it is put to use, and I get the impression you are describing a way to capture ** some ** of all that heat.
(th)
Offline
Like button can go here
#95 2021-11-22 11:01:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
The heat is coming for the reactor that is powering the remaining parts of the digger water processing unit.
https://www.powermag.com/what-are-super … er-cycles/
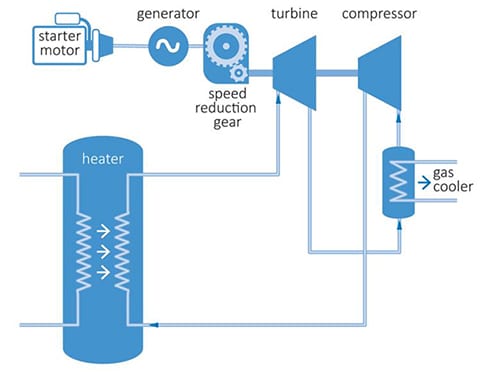
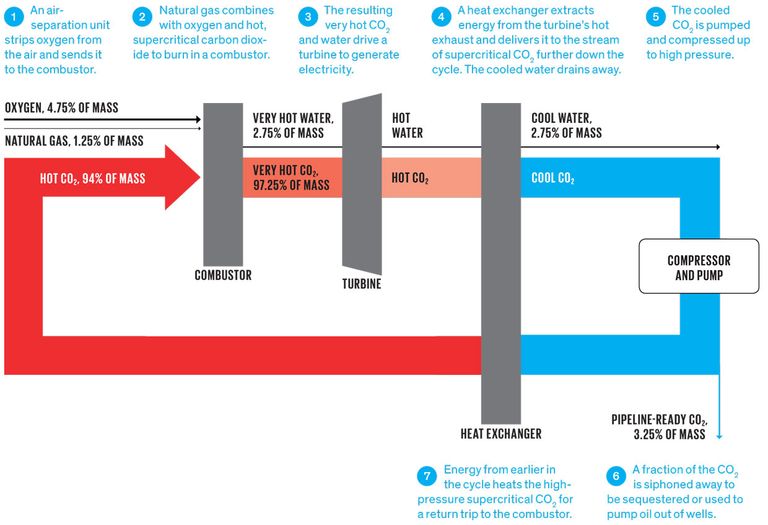
The heater is the chamber where the wash gasses are feed into the turbine compressor combination, where the image of the motor and gears are removed. The outlet is to use a ventui to take and inlet of mars co2 to mix with the exhaust from the turbine where its collected. With the compressed co2 from the mars air into a tank to pressurize and liquefy.
https://netl.doe.gov/sites/default/file … chogen.pdf
Modeling and Experimental Results for Condensing Supercritical CO2 Power Cycles
Offline
Like button can go here
#96 2021-11-22 11:34:53
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut ... thanks for continuing development of this interesting technology presentation ...
I still don't have time to read all the links, but ** do ** have time to point out that nothing you have presented so far shows where the energy to compress the gas is coming from, let alone where the energy to heat the fluid after compression is coming from.
The presentation so far seems to show a mechanism for using CO2 in place of water as a working fluid for a mechanical power system.
Is there a diagram that shows the entire system, from heat source through light bulb output device?
(th)
Offline
Like button can go here
#97 2021-11-22 13:34:09
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,761
Re: Mars Water regolith soils 1 foot depth only
Heat driven expansion of the wash gas is used to turn the turbine with a common shaft to the compressor that is used to draw the mars air in. We are not making electricity at this time as we are making use of the thermal energy to drive the process of collection.
So far the reactor has plenty of electrical to drive the inlet fans that start the collection to feed the baking chamber with the wash gas and vapor from the heating process. The compressor is used to add more to the collected volumes.
To boost the critical we can wrap the line around the core to further boost the pressure that is going into the turbine.
https://link.springer.com/article/10.10 … 020-2116-6
https://web.mit.edu/22.33/www/dostal.pdf
A Supercritical Carbon Dioxide Cycle for Next Generation Nuclear Reactors
Offline
Like button can go here
#98 2021-11-22 16:54:58
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,212
Re: Mars Water regolith soils 1 foot depth only
SpaceNut, that is an interesting idea. What you are describing sounds a bit like a high bypass turbofan engine with a nuclear heat source. In this case, all of the gas turbine work output is consumed by the compressor, but much of the compressor power is used to compress the bypass fraction, which is liquefied. The neat thing about this is that you don't need heat exchangers, which would add a huge amount to mass budget. At Martian temperatures, the cold CO2 gas requires very little compression work to compress it to a high density mixture of gas and CO2 droplets. This improves the efficiency of the gas turbine, because it allows a very high expansion ratio. The bypass fraction may require some heat exchangers, due to the heat of fusion that must be absorbed as it phase changes back into a liquid. Alternatively, you could centrifuge the compressed, two phase mixture, draw off the liquid and pass the dry gas into the heat source and through the turbine.
Compressing the Martian air like this would also produce a steady amount of water. In fact water may be a problem, as ice may plate out on blades and surfaces as pressure increases. We may need some sort of inter-stage condenser that it can dew out onto.
One limiting factor in this scenario is low atmospheric density. With a density of just 13 grams per cubic metre and windspeed <10m/s for much of the year, the turbine would need a large swept area relative to its output.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#99 2021-11-22 17:17:56
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mars Water regolith soils 1 foot depth only
Why does it have to be that short a time? Won't the stay be more like 18 months minimum - a minimum of 500 sols I would think.
For SpaceNut re #71
You need to develop a continuous process to deliver 1200 tons of propellant in 365 Earth Days.
Edit: Corrected 2021/10/20 from 2400 .... the original quantity of 1200 tons was given by GW Johnson earlier in this topic.
This would be slightly fewer Sols.
My recommendation is to design a plan that feeds output continually from one stage to the next.
If you have to operate in batches, then each batch from one operation needs to feed automatically into the next.
Multiple lines can (and obviously must) be operating in parallel.
(th)
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#100 2021-11-22 18:14:42
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,969
Re: Mars Water regolith soils 1 foot depth only
For Louis re #99
Thank you for an excellent question.
It provides an opportunity for clarification.
The fuel must be prepared and in storage ** before ** a human leaves Earth.
The scenario you are imagining is that a crew would land on Mars without having return fuel already in place.
No prudent mission planner would take such a risk.
To the best of my knowledge (admitting it is limited) NO responsible author has suggested sending a crew to Mars without already having the return fuel and oxidizer in place.
(th)
Offline
Like button can go here