New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2021-10-02 19:07:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Mars Water regolith soils 1 foot depth only
Lets keep to topic related of energy to gather, process and save quantity for a starships return.
Sub topics are energy to Co2 to gather, pressurize or condense to liquid, unusual properties of CO2 when it’s compressed by pressure in excess of 73.8 bar at temperatures higher than 31° Celsius. Under these conditions its consistency is as thick as a liquid, but it flows as easily as a gas with energy to separate bonds to co +o possible answer on earth in a RGWS:

The equipment is to modify the climework units which the blowers are most likely at or under 500 rpm to the same as the mars helicopter capable of 3 to 4000 rpm as that would allow a blower of co2 compatible to the units here on earth for mars use. The remaining part of the unit is untouched to perform the same act as we would on Mars.
https://en.wikipedia.org/wiki/Climeworks
capture about 900 tons of CO2 annually Fans push air through a filter system that collects CO2. When the filter is saturated, CO2 is separated at temperatures above 100 degrees Celsius.
One could also use a thermal chimney updraft to allow for the cold co2 to be forces up the chimney where it can be concentrated with a steady decreasing of size causing a rise in pressure which will allow for an easier compression and collection of the much needed gas.
Using a source of heat under the shape will accelerate the draw and updraft.
NASA and DOE to test kilopower nuclear reactor for space applications thermal chimney post
https://peer.asee.org/student-project-t … -power.pdf
https://en.wikipedia.org/wiki/Solar_chimney
https://en.wikipedia.org/wiki/Solar_updraft_tower
https://www.solaripedia.com/13/371/5041 … ssion.html
https://www.solaripedia.com/files/972.pdf
https://architecture.mit.edu/sites/arch … roject.pdf
https://en.wikipedia.org/wiki/Concentrated_solar_power
solar power towers on mars - km-high vertical wind tunnel turbo-elec
Solar chimeys - Feasible?
Solar thermal power - Fathers Day gift
An Empirical Model of Thermal Updrafts
You need to use a different design for CO2 capture on Mars, like the Dyson vacuum design (centrifugal force), to spin the dust out of the air so that the CO2 entrainment portion of the device (the zeolite bed) is not caked with fine dust. I wonder if there's a liquid polymer that could capture CO2. That would also solve the problem of dust capture, because a filter media could remove the dust contamination from the polymer.
cyclone technology or the act of particulate separation...
https://en.wikipedia.org/wiki/Cyclonic_separation
https://www.processingmagazine.com/mate … iderations
https://mdpi-res.com/d_attachment/proce … 521-v2.pdf
https://www.ajer.org/papers/v5(04)/O050401300134.pdf
Something to look at if energy and amounts work.
We also need to do the co2 freezer calculation as well to get to the magic number as its still a frost on a surface where the chamber contents only 70% are deposited over a period of time onto the freezer plate.
post 38 of the topic contains information.
Sticking to the original topic of Moxie, I note that the Martian atmosphere already contains sparse concentrations of CO and O2.
https://en.m.wikipedia.org/wiki/Atmosphere_of_MarsAt typical Martian temperatures, CO2 is close to its triple point temperature of ~220K. This makes CO2 compressible with relatively little pumping power. With the CO2 removed as a liquid, we could then cryogenically cool the remaing N2, Argon, CO and O2, until they selectively liquefy. Hence, without any chemical reactions, we could extract CO and O2 from the Martian atmosphere. Making methane can then proceed using the reverse gas shift reaction to produce hydrogen, which is then reacted with CO.
I would propose using an axial compressor to compress the Martian atmosphere up to pressures of 5 bar and then a piston compressor, which will compress and liquefy the CO2, with each cylinder having a drain. The axial compressor should face into the wind to ensure a steady flow rate into the fan. The liquid CO2 should be vapourised using waste heat from the KRUSTY and used to drive the axial compressor by passing the hot gases through a gas turbine. The axial compressor will need to be started using an electric motor.
Average wind speed on Mars is 10m/s and atmospheric density in the northern hemisphere is about 0.015kg/m3. That equates to an average of 0.015kg/m2/s incident on each square metre of the turbofan inlet. Of this, 0.075% by volume is CO. This amounts to 0.07 grams / m2 /s. A circular inlet, some 2m in diameter, would gather an average of 0.22 grams / second. That is 7 tonnes per year. How much CO do we need, to make all of the propellant needed by the Starship?
https://www.netl.doe.gov/sites/default/ … ok/2-0.pdf
https://marspedia.org/Atmospheric_processing
That KBD512 and Caliban have spoken of with regards to power using super critical heated co2. Its a turbine driven system using the heated co2 to make motion and on the other side its cooled and brought back to the cycle once more.
Sub topic to take gather water and energy to break it bond into H + O of which the next step is to pressurize or condense to liquid.
If you are looking for hydrogen to be a savior a gallon of gas is equal to 1 kg of hydrogen and to get that you need to break down 2.64 gallons of water to obtain the equivalent in hydrogen as there is only 110 grams of it in a liter of water.
For earth that is not a problem as we use free air in the engines of choice but for mars we need to capture the oxygen for reuse.
It is also said that the electrolysis input to get the hydrogen is just 70% efficient.
https://www.energy.gov/eere/fuelcells/h … ectrolysis
Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700°–800°C, compared to PEM electrolyzers, which operate at 70°–90°C, and commercial alkaline electrolyzers, which typically operate at less than 100°C). Advanced lab-scale solid oxide electrolyzers based on proton-conducting ceramic electrolytes are showing promise for lowering the operating temperature to 500°–600°C. The solid oxide electrolyzers can effectively use heat available at these elevated temperatures (from various sources, including nuclear energy) to decrease the amount of electrical energy needed to produce hydrogen from water.
The electrolysis process uses between 40-50kWh to generate 1 kg of hydrogen. So, using the lower end of that range, the electric power required for 250 kg of H2 is about 10 mWh, which, when scaled up to the full launch amount, is about 10000mWh, or roughly 415 mw of generating capacity working 24 hrs a day is required to supply the hydrogen for a single launch.
Many think that under the ice cap for some craters is a salty water just waiting for the use by man.
This ‘brine electrolyzer’ can mine oxygen, hydrogen from water on Mars
Engineers at Washington University (WashU) in St. Louis
Mars, though, the water contains a fair amount of magnesium perchlorate — salt and their system work better since such high concentrations of salt keep water from freezing on such a cold a planet by lowering the liquid’s freezing temperature to -60 °C.
NASA’s current mandate is to land humans on Mars by 2033. Here, we demonstrate an approach to produce ultrapure H2 and O2 from liquid-phase Martian regolithic brine at ∼−36 °C. Utilizing a Pb2Ru2O7−δ pyrochlore O2-evolution electrocatalyst and a Pt/C H2-evolution electrocatalyst,
To satisfy those same oxygen requirements, the cell active area of the 2.2V brine electrolyzer is .375m and 1.2m^2 respectively.
The hype is for the oxygen at a greater amount than that of a moxie but the process is for the hydrogen since we can not make fuel without it.
A human requires around 550 Liters of pure gaseous oxygen per day.
Sub topic Sabatier reaction to produce methane energy needs and liquification.
METHANE SYNTHESIS USING A SABATIER-ELECTROLYZER
https://learn.openenergymonitor.org/sus … er-process
Power-to-Gas – A technical review and in German (El-till-Gas – System, ekonomi och teknik)
Based on the information in the flow chart presented later, the estimate that it takes about 8.1 kWh per kg of methane to drive a Sabatier process.
sub topic energy needs to refuel starship
Which is a geological survey to assure the surface structural support and water is available for a crew once there. Of course this fits well with Oldfart1939's crew of 17 mission structure to leverage the site to its fullest on its first crew landing.
companion site discussion only author of the main page having sole content rights to make the plan from discussion.
This is a 2 cargo starships and one crew at the selected site. This is the mars Toe or Foot hold we are looking for to make mars sustainable. Any misjudgment of water or landing is going to play big in cancelling the future to mars.
This will allow for 2 types of payloads between 100 mT to 150 mT depending on fast or slow transit to mars.
All initial numbers are working for single reference points as we can make changes that influence them via the fact we will have 2 cargo ships payload capacity to make use of.
Next up is the crewed mission which could further have 2 additional cargo ships in the same launch window for mars landing site.
Offline
Like button can go here
#2 2021-10-03 11:09:28
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,131
- Website
Re: Mars Water regolith soils 1 foot depth only
"Any misjudgment of water or landing is going to play big in cancelling the future to mars."
THAT is exactly what the engineering lander is supposed to prevent.
GW
GW's oldfarts1939 topic starship fuel numbers these are to leave earth with given payloads and time periods to mars as well as returning home from mars.
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#3 2021-10-03 11:18:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Adding to the water ice locations thanks
We don't need to transport water over long distances. Instead build your city next to a major deposit of ice.
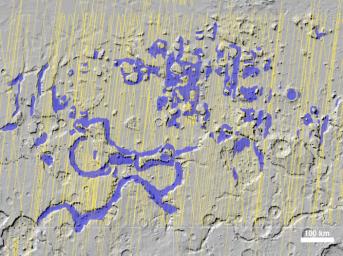
Glaciers discovered by the shallow radar instrument called SHARAD on Mars Reconnaissance Orbiter.
This map of a region known as Deuteronilus Mensae, in the northern hemisphere, shows locations of the detected ice deposits in blue. The yellow lines indicate ground tracks of the radar observations from multiple orbits of the spacecraft.
The ice, up to 1 kilometer (0.6 mile) thick, is found adjacent to steep cliffs and hillsides, where rocky debris from slopes covers and protects the ice from sublimation into the atmosphere.
The base map of this image is shaded relief topography obtained by the Mars Orbiter Laser Altimeter on NASA's Mars Global Surveyor. The image is centered at 42.2 degrees north latitude and 24.7 degrees east longitude. It covers an area 1050 kilometers by 775 kilometers (650 miles by 481 miles).
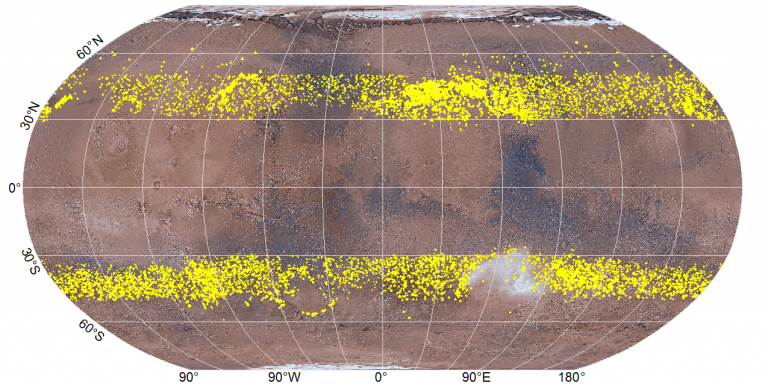
A global map of Mars showing the distributions of features thought to be debris-covered glaciers (shown in yellow) in Mars’ mid-latitude regions. The basemap is made of colour images from the Viking 1 and Viking 2 orbiters. The glacier distributions are from maps by Levy et al. 2014 3 and Souness et al. 2012
A glacier-like form in Mars’ northern mid-latitudes (42° north, a similar latitude to northern Spain on Earth). It is approximately 8 km long. This perspective view was generated from 25 cm resolution images taken by the High Resolution Imaging Science Experiment (HiRISE) camera on the NASA Mars Reconnaissance Orbiter spacecraft. The HiRISE image is draped over a 3D surface which was generated from two ‘stereo pair’ images taken from different angles by the spacecraft.
A lineated valley fill in Mars’ northern mid-latitudes. The inferred direction of past ice flow is from the bottom to the top of the image. The main trunk is fed by two large tributary glaciers. Image mosaic from the Context Camera on Mars Reconnaissance Orbiter.
A ‘concentric crater fill’ type glacier occupying an impact crater in Mars’ northern mid-latitudes. The distinctive concentric surface flowlines give these features their name. In this example, the glacier has almost completely infilled the crater; only the rim of the crater is visible. Image mosaic from the Context Camera on Mars Reconnaissance Orbiter.
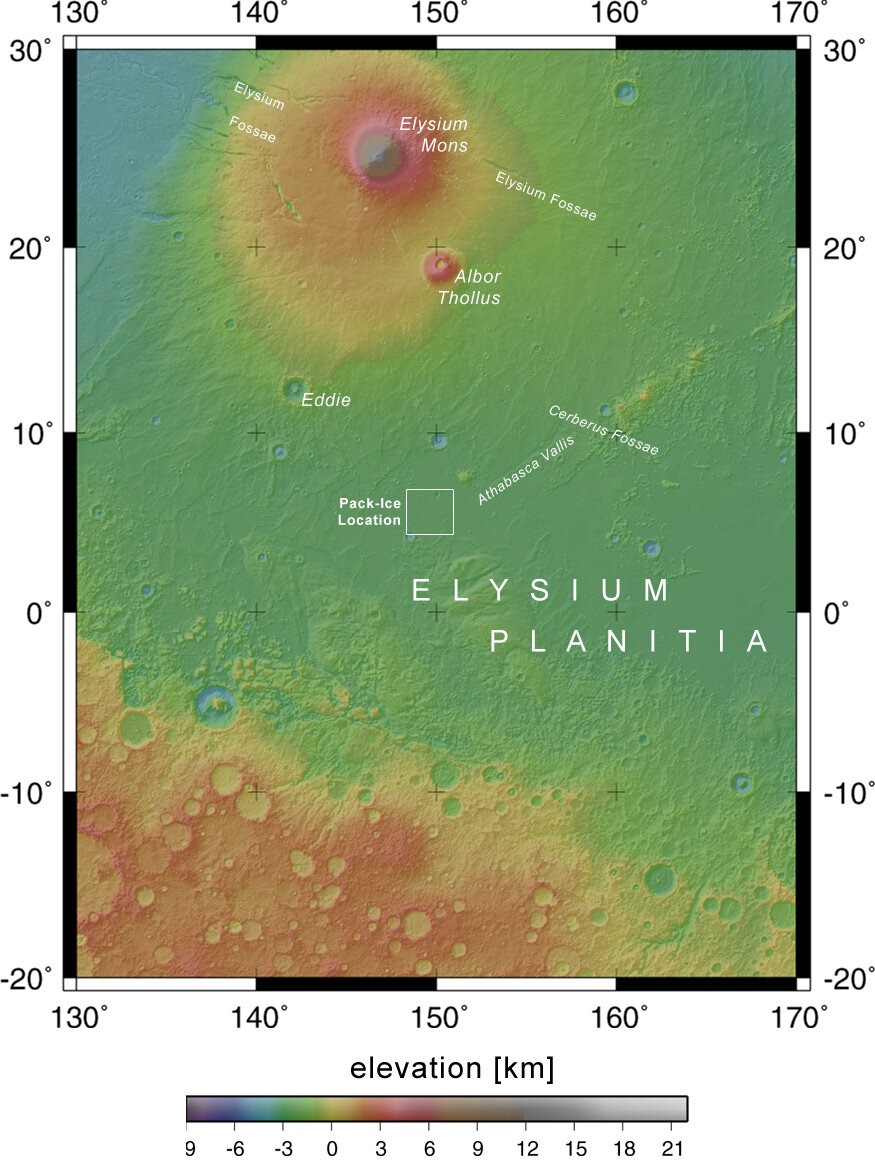
Most people want a location close to the equator. Mars is cold so you want the warmest possible location. There's also the "frozen pack ice" of Elysium Planetia.
From the European Space Agency: ESA’s Mars Express sees signs of a ‘frozen sea’This image, taken by the High Resolution Stereo Camera (HRSC) on board ESA’s Mars Express spacecraft, shows what appears to be a dust-covered frozen sea near the Martian equator.
It shows a flat plain, part of the Elysium Planitia, that is covered with irregular blocky shapes. They look just like the rafts of fragmented sea ice that lie off the coast of Antarctica on Earth. This scene, taken during orbit 32, is a few tens of kilometres across, and is centred on latitude 5º North and longitude 150º East.
The water that formed the sea appears to have originated beneath the surface of Mars, and to have come out through a series of fractures known as the Cerberus Fossae, from where it flowed in a catastrophic flood.It collected in a vast area about 800 kilometres long and 900 kilometres wide with a depth of about 45 metres. As the water started to freeze, floating pack ice broke up into rafts. These became later covered in ash and dust from volcanic eruptions in the region.
Above image is from New Scientist, taken by High Resolution Stereo Camera on Mars Express. It shows "sploosh" craters. They can only form by meteorite impacts on ice. The perfectly smooth bowl of the crater is ice melted by the impact. The floor of the crater in the centre is flat and rough, that's the ground beneath the ice. And a small pit in that flat floor is where the meteorite impacted. The ridge around the rim is where slush splashed up and froze. These craters show scientists depth of the ice, and is how ESA scientists confirm the ice is still there.Note: the volume of ice in the "frozen pack ice" is greater than all the water in the Great Lakes combined, and depth is the same as Lake Erie. Or equal to the water of the North Sea.
I found I had posted about test
https://www.nasa.gov/sites/default/file … elease.pdf
Which had quite a few TBD indicators throughout the paper...Nasa is still trying to develope a system for mars whie running tests in
Antartica which is no mars...
First is the low gravity which will allow vapor to transistion from ice with only a slight bit of heat from the drilling and then as it rises its going to refreeze binding the drill. So we will need to place a dome to seal the drilling and pressurize to keep the water in liquid form.
Its the level of confirmed water levels which need to show way more than just a single landing refueling at were we do the testing.
We will need to know over a very large grid as to if the water fades as we go from a high density location.
We are trying to select a sustainable location in which we can do more than a sortie science mission as a crewed follow up.
Offline
Like button can go here
#4 2021-10-03 11:58:05
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re #221
The current plan (as I understand the work of GW Johnson) is to deliver ONE fixed site drill to go down 10 meters to look for water, AND ONE rover to pound the regolith over a considerable surface around the lander with the drill.
The purpose of the existing plan is to determine if there is any water at all at the chosen location (a) and (b) to establish the character of the regolith ahead of a landing by a rocket capable of return to orbit.
In your post #221, you have raised a question that is most certainly above **my** pay grade, and which deserves a serious answer.
I think the underlying concept you are expressing is wrong, but ** I ** could be wrong.
As I read your post, you are imagining that drill might find water in a column directly below the lander, and that there would be NO other water for hundreds of meters around the bore hole.
What you've described seems (to me at least) to defy the laws of physics.
However, fortunately we have members of the forum who can address the probability of water in a location if measured with only one drill operation.
This is ** not ** intended as anything other than a call for clarification by those who can assist SpaceNut with his question.
The practical consequence (if SpaceNut is right) would be that the drill would have to be designed to drill more than once at a site.
Such a modification is NOT out of the question. GW Johnson has been leaning toward a vision of 12 (or so) small lander packages to drop in on 12 (or so) sites of interest. If your theory that water could exist under a lander but nowhere else is correct, then the lander could be increased in mass, and there would be fewer of them.
Or! A given site could "enjoy" a visit by two landers instead of just one.
However this turns out, thanks for a provocative and interesting challenge for GW Johnson (and others) to consider.
(th)
Offline
Like button can go here
#5 2021-10-03 14:28:13
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
This is simular to the drilling of a well at ones property here on earth in that if you need a towns supply due to starships needs and you come up with a neighborhoods of capability you fail for mars. You will not only exert more time to find more water while on the first mission but stay in that mode for that site as the resource is not there to which we need.
probable landing sites
orbital measurements with MRO readar
watery past
New water map of Mars will prove invaluable for future exploration

Offline
Like button can go here
#6 2021-10-03 15:00:56
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
https://www.extremetech.com/extreme/167 … sity-rover
If you heat up a cubic foot of Mars soil, you can harvest around two pints (one liter) of water. According to new data returned by NASA’s Curiosity rover, this isn’t just a one-off lucky find, either: It seems that most of the dirt on Mars harbors large amounts of water.
1 cubic ft = 0.02831685 cubic meter
1 cubic meter = 35.3146667 cubic ft
Weight of 1 liter (l) of pure water at temperature 4 °C = 1 kilogram (kg). A liter is defined as one cubic decimetre (1 l = 1 cubic dm). 1 l = 1kg of water = 0.001 cubic m = 0.264172052 US gallons = 1.05668821 US quarts.

Its unknown if the concentration weens off with depth..so thats why we need a mobile rig that can get deeper than a meter.
Baking soil
Use of a microwave oven for moisture determination in a soil science laboratory
probably not for mars as its testing is grams with low concentration for time to dry from 5 minutes to 30 but possible.
Sterilizing is similar to what we are doing
Prepare to Bake Soil
Preheat the oven to 200 degrees Fahrenheit. Spread the bottom of a metal or glass baking pan with 4 inches of soil, advises Oklahoma State University Extension. Do not press the soil down; leave it loose. Cover the baking pan with aluminum foil.
Monitor Soil TemperatureSlide the pan into the oven and check the temperature every 15 minutes. One option is to poke a meat or oven thermometer through the foil and into the soil. Otherwise, just use a cooking thermometer to check the temperature.
Baking soil gives off an odor that some people find unpleasant. To avoid filling the kitchen with this smell, bake the soil on a nice day so the kitchen windows and door can be left open. If a strong, sharp smell comes from the oven, check the temperature of the soil. Usually this means that the temperature has suddenly risen beyond 200 degrees Fahrenheit.
Finish Sterilizing SoilShut off the oven when the soil reaches 150 degrees Fahrenheit. Let the pan rest in the warm oven for half an hour. The temperature of the soil will keep rising to about 180 degrees Fahrenheit, advises Oregon State University Extension Service.
Crack the oven door if the soil temperature reaches 200 degrees or above. Baking the soil at too high a temperature cooks the soil and begins to destroy the structure
https://www.gardeningknowhow.com/garden … g-soil.htm
Sterilizing Soil with an Oven You can also use the oven to sterilize soil. For the oven, put some soil (about 4 inches (10 cm.) deep) in an oven-safe container, like a glass or metal baking pan, covered with foil. Place a meat (or candy) thermometer into the center and bake at 180 to 200 degrees F. (82-93 C.) for at least 30 minutes, or when soil temp reaches 180 degrees F. (82 C.). Anything higher than that can produce toxins. Remove from oven and allow to cool, leaving the foil in place until ready to use.
So time to dry and drive out moisture is dependent on content amount.
Offline
Like button can go here
#7 2021-10-03 16:46:57
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
I have with Oldfart1939 done some this before and will add it once found.
To it such that we assume this is the ground proof found until we can drill in multiple location on a selected landing site to greater depths.
Back to the water and radiation protection its just 15 cm depth and if that is frozen mud all that is needed is to cover it with a seal to keep the protection in place. I wonder if thats close to the 1 ft 1 liter amount...
research found that 1 liter is 1000 cm^3 or a 10 cm cube
so a 1 ft is 30.48 cm making the need 9 liters to gain the radiation protection when mixed with regolith
Here is one of the topics Practical Water Extraction Methods for Starship Missions which contains some of what I am looking for.
another Foot hold for manned missions
contains soil composition Mars soil good for crops for getting a good start on sustainability
edit
adding posts for baking soil for water
Water Extraction from Martian Soil
Previous exploration missions to Mars, such as the Viking missions, have determined that water can be extracted from the soil if the soil temperature is increased to between 200°C and 500°C. These same missions also determined that water is present in the soil at approximately 2% by mass. Research has determined that the water in the soil can be recovered when the soil is heated to a temperature between 200C and 500C.
https://www.sbir.gov/node/1227055
For hydrated soils, the excavated soil can be delivered to a centralized soil processing plant or processed on the excavation rover itself. The amount of water content in the hydrated Mars soil can vary from as low as between 1.5 and 2% on the surface at almost all locations on Mars to above 10% depending on the location and mineral.
Bonus water from the heating to break down.
https://mepag.jpl.nasa.gov/reports/Mars … _Study.pdf
https://ntrs.nasa.gov/api/citations/201 … 010258.pdf
Extraction and Capture of Water from Martian Regolith Experimental Proof-of-Concept
https://kiss.caltech.edu/workshops/isru … ders_2.pdf
Mars Water Mining for Future Human Exploration
Slides hold the movement equations which show how we waste time for some activity.
Offline
Like button can go here
#8 2021-10-04 19:59:46
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Starship needs 800 mT to 1100 mT for the return trip home depending on how large a payload we bring home.
2(H2O) = 36 /2 is H 2 and Oxygen 16 to make the mole mass.
1 liter or 1 kg is 2( H = 56 grams, Oxygen = 448 grams) so rounding up error.
chemical process known as the Sabatier process,
Carbon dioxide (CO2) and Hydrogen (H2) would create Methane (CH4). CO2 + 4H2 = CH4 + 2H2O.
https://nssdc.gsfc.nasa.gov/planetary/m … ssurf.html
The ISRU module will react imported hydrogen with atmospheric carbon dioxide in order to achieve this. For each metric ton (tonne) of imported hydrogen that is reacted, 2 tonnes of methane and 4.5 tonnes of water will be produced. The electrolysis of 4.5 tonnes of water will yield 4 tonnes of oxygen and 0.5 tonnes of hydrogen which can be recycled back into the Sabatier process
CO2 is 12 + 2(16) = 44 grams
4H2 is 4(2) = 8 grams
CH4 is 12 + 4 = 16 grams
2(H2O) is 2( 2 + 16) = 36 grams
combustion of 2 CH4 + 3 O2 ———> 2 CO2 + 4 H2O
3o2 is 3(2(16)) = 96 grams
2(CH4) is 2(12 + 4) = 32 grams
2 (co) is 2(12+8) = 40 grams
4 (H2O) is 4 ( 2+16) = 72 grams
other ratio's will have a different equation for combustion
1 mT = 1,000 kg or 1,000,000 grams
When the vehicle is vertical, the larger liquid oxygen tank is on the bottom and the smaller liquid methane tank is on the top. ... The reason for the different sizes in tanks is due to the mixture ratio between methane and liquid oxygen Oxidizer vs. Fuel Ratio 3.7 : 1 or oxygen and methane mixture ratio would be at 3.6 to 1.
The Super Heavy Starship uses subcooled fuel, which allows the rocket to carry 10 to 12% more of it in the same volume and will contain 4,800 tons of propellants (78% oxygen & 22% methane). with starship getting 240 mT of methane with oxygen 860 mT with the remaining being used in the first stage BFR.
now to work back to how much co2 is from atmosphere and how much is water recovered from the ground 1ft
https://www.nyserda.ny.gov/-/media/File … is-PDF.pdf
Offline
Like button can go here
#9 2021-10-04 20:16:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re entire topic ...
Thanks for working on this series!
One thing might help ... it is (probably) not necessary to super cool the fuels for the Starship leaving Mars.
The .38G to be overcome may allow for "normal" cooling.
I bring this up because you are going to need a lot of energy to cool both the fuel and oxidizer, and you're going to consume energy keeping the two fluids liquid for months, while you build up enough material for a launch.
I don't know how much energy is required to achieve the supercooled state for these gases, but I'll bet that if that state is NOT required, your're going to be glad to save the energy to make fuel in the first place.
Dr. Zubrin recommended a nuclear reactor for the energy to prepare for a launch, at the scale you've shown in the posts above.
The energy required to make fuel, to chill it to liquid, and to keep it chilled for months on end should be knowable.
From that you can make a reasonable estimate of the size reactor needed to be safely delivered to the landing site.
(th)
Offline
Like button can go here
#10 2021-10-04 20:41:34
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Yes super chilling would save on energy and while it will mean less fuel in the tanks once filled there is plenty of margin even for that slight change.
https://marspedia.org/Sabatier/Water_El … is_Process
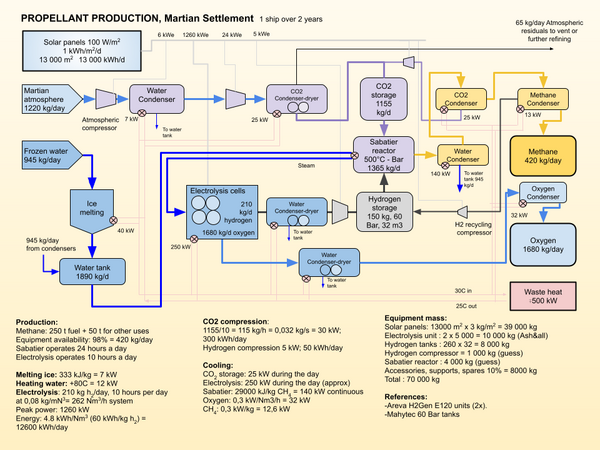
https://ntrs.nasa.gov/api/citations/201 … 004697.pdf
Sabatier System Design Study for a Mars ISRU Propellant Production Plant
https://marspedia.org/Atmospheric_processing
How they are processed out of the wash after heating the regolith to bake them out.
The first gas to condensate out is water, at 40°C for 10 kPa pressure. After an increase in pressure, the hot dry gas is cooled further to condense the CO2 , at -57°C for 520 kPa. The condensed liquid CO2 can be removed by gravity. Nitrogen at 510 kPa will liquefy at -170°C, and Argon at about the same temperature, so care must be taken if we want to keep these two gases separate. The next gas to condense out is Oxygen.
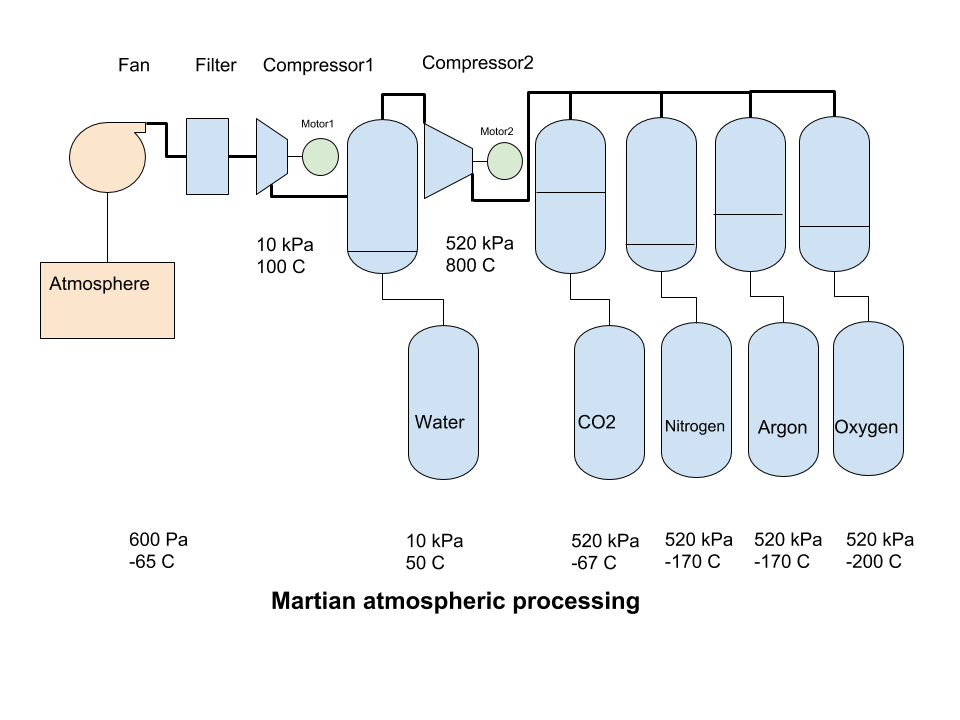
Compression from the initial atmospheric pressure of 600 Pa to 0,1 bar (10 kPa, or 1/10 Earth atmosphere) heats the atmosphere to 100°C but also creates adequate conditions for the condensation of water when it is cooled to about 40°C. It can then be collected at the bottom of a pressure vessel and removed.
Design analysis and trade space and cost estimation (fairly comprehensive):
Actual working hardware analysis (real honest-to-goodness "shipping container mobile" industrial scale hardware):
Actual working synthesized CH4 plant (European- the Falkenhagen P2G Plant, commissioned in 2017):
Innovative large-scale energy storage technologies and Power-to-Gas concepts after optimisation
Believe it or not, the Europeans have already "bellied up to the bar". ESA supplies service modules to NASA, so why not P2G, as well?
Offline
Like button can go here
#11 2021-10-05 06:25:38
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re this topic...
If I understand the premise of your topic here correctly, you have noted that there may be water chemically bound to the regolith wherever we land on Mars, and that it would be possible to free those molecules by heating the regolith.
In earlier posts in this series, you have shown the start of calculations that should yield a figure for the amount of regolith needed to free up enough water to make the methane and oxygen needed to fill a Starship for return to orbit.
** This ** post is about the physical manipulation of all that regolith (a) and ...
(b) what might be done with the other materials that come out of the heating process and ...
(c) what mechanical equipment is needed to scoop up all that regolith and move it to the heating facility and ...
(d) what mechanical equipment is needed to move the results of the heating process to the collection points
It's been so long since I looked at Dr. Zubrin's book on the subject, I've forgotten what his solutions might have been.
If you have a copy (or can find it in your library), it would be interesting for this topic to see what he suggested.
It seems to me you are going to come up with a list of impressive machinery.
All that machinery is going to need power in great abundance to perform their functions for months on end.
It is entirely possible all the work needed to show what is required has been done in this forum in the past 20 years.
It is even possible the work has been done more than once, and your initiative here may be one more repetition.
At the conclusion of all the hard work this new topic requires, I ** hope ** we (forum members and admins) can think of a way to ** try ** to keep it easy to find, so we can use it as a reference and not keep regenerating the content like Groundhog's Day!
(th)
Offline
Like button can go here
#12 2021-10-05 18:28:57
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Since the scouting mission can only drill in one place and the ability for a drilling rover would be limited to just 1 m depth its safe to assume then that we are going to have at a minimum the 1 ft cube just the 1 liter to work on and processing is what I want to stay focused on. This would be what is needed in a first mission of seventeen that Oldfart1939 has started to embark on where in the focus is set up shop and prospect for resources and to expand on that knowledge of a given sites geology. Of course a starship will have more capacity for larger items to be made use of but they are being used to show future capability of use for that site.
We have a baseline with MOXIE or the Co2 gathering and processing with it being scaled up.
We also have sufficient data for the Sabatier reactor capability as well.
Ideal gas law: PV = nRT
P: Pressure
V: Volume
n: number of moles
R: product of Boltzmann constant and Avogadro constant (8.314)
T: Temperature
n = PV / RT
fan blade lift or force would be calculated with is CL ½ p V2 S. where V2 is for velocity or the rotor RPM
https://www.thehelicopterstudyguide.com … -equation/
or this equation
https://sciencing.com/calculate-lift-ro … 80704.html
You suggest -50°C, which is a reasonable temperature. At any location, subsurface temperature will be the average of that spot over time: day/night, summer/winter. So Ok, let's assume -50°C. This is the full triple point chart for CO2: (yes, click image for reference)
So below -56.6°C it's not possible to get liquid CO2; but you said -50. Pressure will have to be a little higher than 0.51 MPa (510 kPa = 74 psi = 5.0 atmospheres). Too much pressure will convert CO2 into solid dry ice. I don't believe we'll find liquid CO2 on Mars.You want more references? Note pressure on this one is logarithmic, and temperature is Kelvin.
Much like the getting of water from dirt the task of getting co2 from a thin atmosphere is also going to be energy intense.
Now back to the water:
So with the 1 liter per cubic ft how large must that field be to get not only fuel water but have an excess for other uses?
Once we have that we can focus on the means to excavate and what that requires.
Offline
Like button can go here
#13 2021-10-05 18:43:46
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For SpaceNut re topic .... congratulations again for your ambition to tackle this important question.
There is one small change I can offer for post #12....
I have no idea where the 1 meter drill depth came from, but the value recommended by Dr. Johnson is 10 (TEN) meters.
The European Space Agency Drill is designed to go down 2 meters, but it is a ** scientific ** expedition.
The drill recommended by GW Johnson is based upon the Canadian model as reported by RobertDyck. As I recall, that drill was designed to go at least 10 meters and it might have been capable of more.
in any case, the target depth for the Lander proposal you have seen is 10 (TEN) meters.
However, this doesn't make any difference for your calculations. You have imagined scraping regolith to a depth of 1 foot, and that seems to me to be quite practical for a front loader (bucket) machine.
Best wishes for success in finding the numbers to refill a Starship in Mars!
(th)
Offline
Like button can go here
#14 2021-10-05 18:47:42
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mars Water regolith soils 1 foot depth only
Its the level of confirmed water levels which need to show way more than just a single landing refueling at were we do the testing.
We will need to know over a very large grid as to if the water fades as we go from a high density location.
We are trying to select a sustainable location in which we can do more than a sortie science mission as a crewed follow up.
10 x 10 x 10 metre of ice is 1000 tons of ice.
I don't think you should exaggerate this problem.
All Space X have to do is send a couple of Starships with a couple of robot rovers on them to explore the neighbourhood and check on water deposits.
If there ain't no water you cancel the human mission.
Simple!
Louis-
The present project is specifically stated to provide sufficient water to produce enough Oxygen and Hydrogen for the return to Earth and also for all other uses, and extracted from ice that's contaminated by who knows what. Your assertions are also without adequate foundations. Getting water that's chemically and physically contaminated into a condition that it's pure enough for electrolysis and human consumption requires a massive amount of processing--AFTER it's been dug out in chunks from strip mining-type operations.
If my method of extraction is used to collect ice, it will be relatively clean. I use the word relatively with caution. My system involves allowing the crude mined ice to melt in a heated environment. The water Probably dirty and possibly even a sludge, will be dewatered with a basket centrifuge that separates the solids from free flowing but still chemically impure water. Distillation is the next step in getting water pure enough for electrolysis and drinking.
Last edited by louis (2021-10-05 18:48:06)
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#15 2021-10-05 18:54:04
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Louis, we are trading adding more equipment to clean the ice before using it in the electrolysis unit continues the grow the size of an already very large power draw.
Oldfarts1939, use of a heat source separate from electrical power is the way to go for the water purification.
Need to look up the ISS Russian waste water to oxygen unit as I remembers the Russian unit does just that spinning to separate the solids from the water.
TH,
A 10 meter drill does not fit into a 5m back shell which is what was used for the MER's.
1 meter is the other drill for the moon.
The esa drill is different in that the center is a pivot point that reduces the through into the ground post 185 in scouting topic has a drilling depth of 1.7 m and 2 meters sideways
This is computing what we know to be true to prove a minimum of what is required. It would be good to see higher levels of depth with the GW work but those are still years away and we can figure this out now. Doing this proves we could go now not wait for the larger vehicle if we send the equipment to the site first and especially if its automated. It also proves the value of doing GW's mission so as to make it easier for the larger vehicle.
Louis we have no ground proof of any thick Ice...
Offline
Like button can go here
#16 2021-10-05 18:56:08
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For Louis re #14
The purpose of the Lander Proposal by GW Johnson et al, is to visit a candidate landing site and investigate to see if there is ANY water there at all, let alone tons of the stuff.
The related purpose of the Proposal is to investigate the characteristics of the lander terrain to see what kind of pads are needed to support any vehicle that might be designed to land on Mars.
There is no point in sending ** any ** heavy craft until that data is in hand.
SpaceNut has taken on a different challenge ... given an estimate that there is chemically embedded water in Mars regolith, SpaceNut is investigating to see how much regolith would have to be baked to release chemically bound water to refuel a Starship.
That is quite a different problem from working with ice, which may ** never ** be found on Mars in any quantity.
(th)
Offline
Like button can go here
#17 2021-10-06 04:25:08
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,363
- Website
Re: Mars Water regolith soils 1 foot depth only
SpaceNut,
I attended the 4th Canadian Space Exploration Workshop at headquarter of the Canadian Space Agency. When was that... 2004? NORCAT had a prototype of their drill on display. It was a multi-segment drill. You know how an oil well has multiple segments for the drill string? Each new section of pipe is screwed into the last. The NORCAT drill does the same thing, but smaller. The NORCAT drill uses an electric motor, and the drill bit is dry, meaning no lubricant required. That makes it applicable for Mars or the Moon. The drill string proposed for Mars would have 10 segments, each 1 metre long. That allows it to fit within an aeroshell for landing. You might think that allows it to drill 10 metres deep, but a portion of the last segment must remain above the surface for the rover to hold and the motor to turn. So expect maximum drill depth 9.5 metres. Later designs increased to 20 segments, so it could drill 19.5 metre depth. And yes, that still means the segments fit within a 1 metre diameter aeroshell. Well, maybe a little more. The proposal in 2004 was for a rover the size of Spirit or Opportunity (MER's), not a big rover like Curiosity.
Offline
Like button can go here
#18 2021-10-06 06:44:41
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,049
Re: Mars Water regolith soils 1 foot depth only
For RobertDyck re #17
Thanks for repeating your post about the 2004 NORCAT meeting, and for adding new detail about the demonstration you saw.
SearchTerm:Drill Canadian design for Mars or Moon
(th)
Offline
Like button can go here
#19 2021-10-07 17:57:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Reply over in the Scouting Mars for Landing Sites topic
Offline
Like button can go here
#20 2021-10-08 19:51:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only

https://curator.jsc.nasa.gov/lunar/lnew … oxygen.htm
https://www.esa.int/Science_Exploration … nto_oxygen
https://www.discovermagazine.com/the-sc … ygen-plant
Splitting Rocks
To separate the oxygen from other components in faux moon rocks, the researchers use a technique called molten salt electrolysis.
By first placing the powdery moon dust into a hot batch of molten calcium chloride salt, then running an electrical current through the mixture, the researchers can let chemistry and physics do the heavy lifting. The oxygen previously trapped in the simulated rocks migrates to an electrode (specifically, an anode), where the researchers can then capture it.
With this technique, the researchers report, they have been able to pull 96 percent of the oxygen out of their imitation moon rocks in the course of just 50 hours. Alternatively, they can extract 75 percent of the oxygen in just the first 15 hours. Plus, as an added bonus, the process leaves behind a mixture of metal alloys that researchers suggest could be useful as well.
For Mars we might be able to use this process
Mars Soil Acquisition and Processing for In Situ Water
The need to excavate down to at least 0.5 meters should be considered. The amount of water content in icy Mars soil can vary greatly as a function of depth and latitude. Based on analysis of Mars orbital data, proposers should assume a minimum of 10% by weight of water/ice up to 90%. Due to human landing and ascent considerations, Mars water based resources should be constrained between +/1 50 deg. latitude. Based on the potential high water content by mass in icy soils, it is expected that icy soil excavated will be either processed in-situ or on the excavation rover itself. Proposers should also assume that up to 0.5 m of soil may exist above icy soils and that excavation down to at least 1 meter is required.
Guess whom's name is in this
https://mepag.jpl.nasa.gov/reports/Mars … _Study.pdf
yup Dale Boucher
The reduction processes, particularly those which use hydrogen as the reducing agent, are the most technologically mature. Oxygen which is chemically bound to iron in lunar minerals and glasses can be extracted by heating the material to temperatures above 900°C and exposing it to hydrogen gas. The basic equation is:
FeO + H2 -> Fe + H2O
This process results in release of the oxygen as water vapor. The vapor must be sepa-rated from the excess hydrogen and other gases and electrolyzed. The resulting oxygen is then condensed to liquid and stored. Experiments using samples of lunar ilmenite, basalt, soil, and volcanic glass have demonstrated the required conditions and efficiency of this process.
I believe this to be correct
Computed 1 meter holds 9 liters approximate to a depth of 0.5 m with the first few inches most likely having little water content
using at least 6 cycles of each equation
Starting with a 200 m x 200 m x 0.5 area creating the seed hydrogen for the first reaction
of course followed to the total of 1200m x 1200m x 0.5m
water gathered to make methane oxygen with co2 mT from the Atmosphere
540mT 240mT 960mT 660 mt
I could be wrong
but now to solve the equipment to use and energy levels next
Offline
Like button can go here
#21 2021-10-09 11:10:47
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Next 2 equipment posts are just place holders for what could be used and not necessarily what will go to mars.
QUOTES FORM TOPIC by Robert dyck and Oldfart1939
Any vehicle would have to be customized for Mars. One suggestion I made was to manufacture the vehicle with titanium alloy instead of steel. Titanium has the same weight (mass), but greater strength.
This is a typical Bobcat skid-steer loader. There are various sizes. This one is S450. ("S" for skid) Rated Operating Capacity: 1,370lb. Operating weight: 5,370lb
This is a typical Bobcat compact track loader. This one is T450. ("T" for track) ROC: 1,490lb. Operating weight: 6,424lb
Track vehicles work better on loose ground and inclines. The track provides more traction. I'm suggesting a compact track loader would be more appropriate for Mars.
An excavator can be compact. The first is E10, Rated Lift Capacity 527lb, Operating weight 2,593lb. The second is E20, RLC 1,098lb, Operating weight 4,306lb.

Bobcat has become known for compact construction vehicles. Other brands manufacture competing vehicles: John Deere, Case, Caterpillar, New Holland, others.
Heavy Equipment Guide: Companies by Skid-Steer Loaders
Battery power construction equipment
I made note that since we do not have a garage for the vehicles when not in use that we will need to solve for how to protect the battery from the mars cold and since we do not wat to waste time we will bring a shelter for each to be packed in.
Construction technology for Mars?
Covering a variety of 3D concrete and other materials to make use of...
Here is another item we might need that already comes in a battery operable
BATTERY 48V 425AH
83 amp external battery charger.
A 240V battery charger is standard
Back a page or so we were talking about how to unload and protect the equipment and I think I have a solution in that we send the equipment in a modified with heat and lighting with side door for crew to enter and exit.

Size them to the equipment that it will store. All that will be needed is to cover them with regolith and connect them into the power and heating systems.
example hook up
We are going to solve to what needs to be the equipment to do the work of gathering the soils and water to be able to get to the goal as soon as we can of a refueled ship.
There is a regolith gathering for lunar use that once altered may be in the list to take as well.
Common Types of Surface Mining Equipment – Sand & Gravel Basics
The most common types of surface mining equipment fall into these categories:
Earth Moving / Construction
Extraction
Material Hauling / Transportation
Processing
Storage / Stockpiling
Shipping
Facilities / Maintenance / OperationsCommon surface mining equipment at a sand and gravel mine can be categorized as follows:
Earth Moving / Construction
Bull Dozers (Dozers)
Backhoes
Graders / Scrapers
Cranes
Skid Steers
Extraction
Front End Loaders (Loaders)
Power Shovels
Bucket Wheels
Draglines
Dredges
Material Hauling / Transportation
Haul Trucks
In-Pit Conveyors
Overland Conveyors
Bins / Hoppers / Feeders
Processing
Screens (Washing and Sorting)
Crushers
Classifiers
Storage / Stockpiling
Stack Conveyors
(Dozers and Loaders to manage the pile)
Shipping
Mix Trucks (Concrete)
Over-the-road Trucks
Trains
Barges
Facilities / Maintenance / Operations
Generators
Fuel Tanks
Water Trucks
Personnel Transport Vehicles (Light Trucks)
Forklifts
Offline
Like button can go here
#22 2021-10-09 11:38:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Continuing with the equipment to mining and process
https://www.nasa.gov/isru/overview
https://www.nasa.gov/feature/nasa-seeks … -resources
estimates, to ship a single kilogram of fuel from Earth to Mars, today’s rockets need to burn 225 kilograms of fuel in transit—launching into low Earth orbit, shooting off toward Mars, slowing down to get into Mars orbit, and finally slowing to a safe landing on the surface of Mars. We’d start with 226 kg and end with 1 kg, which makes for a 226:1 gear ratio. And the ratio stays the same no matter what we ship. We would need 225 tons of fuel to send a ton of water, a ton of oxygen, or a ton of machinery.

NASA studies estimate that a system like this will need to produce about 7 metric tons of liquid methane and about 22 metric tons of liquid oxygen in about 16 months.
Enter RASSOR, or Regolith Advanced Surface Systems Operations Robot, an autonomous mining vehicle designed for one specific purpose: To dig or excavate regolith in low-gravity conditions.
https://www.philipmetzger.com/category/citizen-space/
looks promising for the gathering
I am thinking that the unit which would gather the soils to depth would also convert the soils water as it goes. Placing it into a transfer tank. It could be a smaller KRUSTY powered unit as that would give all the heat required to bake the water content out of the soils as it digs it from the ground and moves it to the chamber and then back out after the water is gone from it.
NASA and DOE to test kilopower nuclear reactor for space applications aka KRUSTY

https://news.engineering.iastate.edu/20 … mpetition/
I am also thinking that the rotary unit could have small scoop shaped cavities to dig and throw the soils into a hopper before its moved inside.
bulk agregate gathering
gavel screening to reduce stone content
Offline
Like button can go here
#23 2021-10-09 20:09:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
Supporting documents
https://www.lpi.usra.edu/publications/r … /csm01.pdf
https://www.hou.usra.edu/meetings/ppw2015/pdf/1026.pdf
https://kiss.caltech.edu/workshops/isru … ders_2.pdf
How to get water on Mars? UW researchers are working on a way to cook it out of soil
Bruckner’s concept calls for placing a dome over the soil, injecting microwaves to heat up the soil particles, liberating the water and capturing the moisture.
So the equipment and energy are still an unknown ...
https://ntrs.nasa.gov/api/citations/201 … 001808.pdf
Mars Atmospheric Capture
https://ntrs.nasa.gov/api/citations/201 … 015862.pdf
Evaluation of Mars CO2 Capture and Gas Separation Technologies
earth based systems taking in power plant exhaust gasses
https://www.powermag.com/capturing-co2- … uefaction/
Offline
Like button can go here
#24 2021-10-09 20:11:57
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,465
Re: Mars Water regolith soils 1 foot depth only
If that European SOLAR-JET experiment ever attains 15% efficiency, then 2km^2 of solar panels could refill a Starship's every 31 days, but it requires input CO2 and H2O, which must be collected and purified on Mars. They're making kerosene, but I presume their process could be adapted to produce Methane, since the first step is Syngas production. The production of the Syngas alone must be more efficient than that.
Offline
Like button can go here
#25 2021-10-09 21:31:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,354
Re: Mars Water regolith soils 1 foot depth only
The chamber uses a different process and much higher temperatures which are present via solar concentration focused through a quartz lens.
http://www.sun-to-liquid.eu/
Fischer-Tropsch synthesis to a mixture of naphtha, gasoline, and kerosene
Offline
Like button can go here

:format(webp)/cdn.vox-cdn.com/uploads/chorus_image/image/69835578/1235138610.0.jpg)