New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2016-11-05 13:29:45
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
November 05, 2016
Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for rockets
On October 5th 2016, Ranga Dias and Isaac F. Silvera of Lyman Laboratory of Physics, Harvard University released the first experimental evidence that solid metallic hydrogen has been synthesized in the laboratory.
It took 495 GPa pressure to create. The sample is being held in the cryostat in liquid nitrogen.
Atomic metallic hydrogen, if metastable at ambient pressure and temperature could be used as the most powerful chemical rocket fuel, as the atoms recombine to form molecular hydrogen. This light-weight high-energy density material would revolutionize rocketry, allowing single-stage rockets to enter orbit and chemically fueled rockets to explore our solar system. To transform solid molecular hydrogen to metallic hydrogen requires extreme high pressures.
Isaac Silvera headed a 2011-2012 NIAC metallic hydrogen project and also co-wrote a 2010 paper on metallic hydrogen rockets.
NOTE Nextbigfuture believes until metallic hydrogen becomes very, very cheap, it will be far more valuable for possible superconducting properties than for rocket fuel. Even very high performance rocket fuel. If metallic hydrogen is a room temperature superconductor that is metastable after releasing the pressure that created it, and the critical current is very high, then improvements to engines and high performance magnetic sails would be possible. This will be considered in future posts.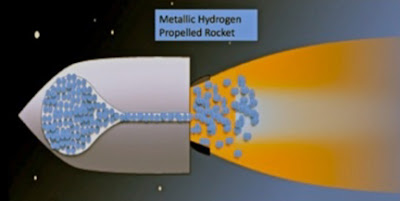
Metallic Hydrogen: The Most Powerful Rocket Fuel Yet to Exist Isaac F. Silvera and John W. Cole
There was a 22 page presentation in 2012 on metallic hydrogen as a rocket fuel
Some Remarkable Properties of Metallic Hydrogen
•Recombination of hydrogen atoms releases 216 MJ/kg
•Hydrogen/Oxygen combustion in the Shuttle: 10 MJ/kg
•TNT 4.2 MJ/kg
•Theoretical Specific Impulse, Isp
•Metallic Hydrogen 1000-1700s
•Molecular hydrogen/oxygen ~460 s (space shuttle)
•Metallic density
about 12-1315 fold of liquid molecular hydrogen [lab results of actual metallic hydrogen was 15 times denser]
•Sufficient thrust for single-stage to orbit; explore outer planets
Silvera Goals
•Produce metallic hydrogen in small quantities [now this is done in 2016]
•Test for metastability [next on the objectives]
•Determine Critical Temperature for conversion
•Develop a method to scale down the critical pressure [Silvera has ideas about injecting electrons]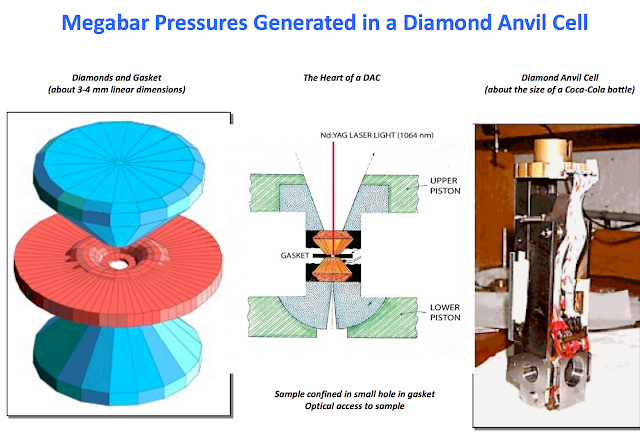
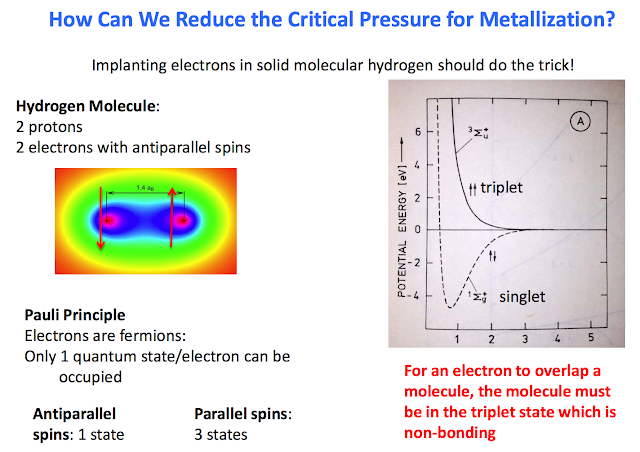
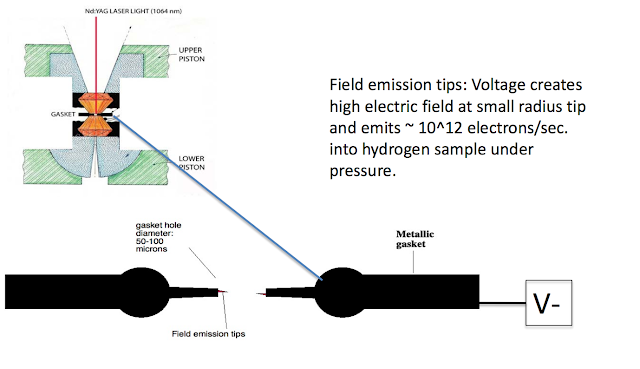
It was predicted that metallic hydrogen might be a metastable material so that it remains metallic when pressure is released. Experimental pressures achieved on hydrogen have been more than an order of magnitude higher than the predicted transition pressure and yet it remains an insulator. Tthe applications of metastable metallic hydrogen to rocketry. Metastable metallic hydrogen would be a very light-weight, low volume, powerful rocket propellant. One of the characteristics of a propellant is its specific impulse, Isp . Liquid (molecular) hydrogen-oxygen used in modern rockets has an Isp of ~460s; metallic hydrogen has a theoretical Isp of 1700 s! Detailed analysis shows that such a fuel would allow single-stage rockets to enter into orbit or carry economical payloads to the moon. If pure metallic hydrogen is used as a propellant, the reaction chamber temperature is calculated to be greater than 6000 K, too high for currently known rocket engine materials. By diluting metallic hydrogen with liquid hydrogen or water, the reaction temperature can be reduced, yet there is still a significant performance improvement for the diluted mixture.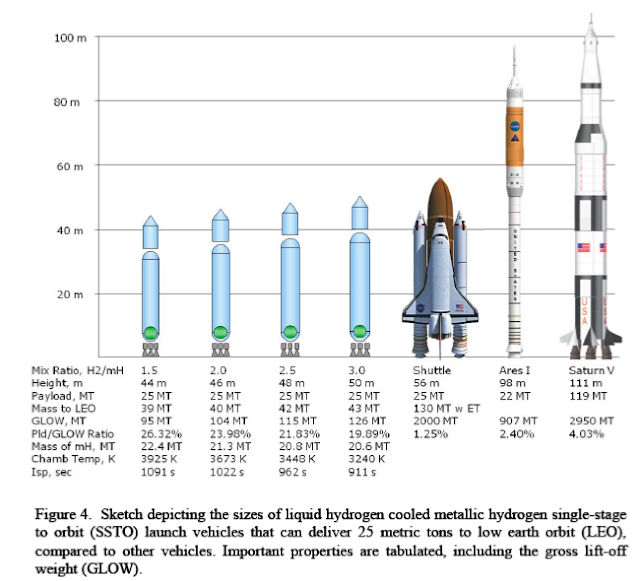
http://www.nextbigfuture.com/2016/11/co … wrote.html
Last edited by Tom Kalbfus (2016-11-05 13:30:33)
Offline
Like button can go here
#2 2016-11-06 10:51:56
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Interesting!
I wonder what the effect of introducing deuterons or alloying elements would be.
Offline
Like button can go here
#3 2016-11-06 11:23:54
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
I wonder how you would set of metallic hydrogen so that it explodes! It the stuff is stable at room temperature and normal pressures, what destabilizes it? The paper wasn't very specific. Lets say you had a kilogram of metallic hydrogen sitting on the table, and you touched it with your finger. Does it explode!
Offline
Like button can go here
#4 2016-11-06 11:33:42
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
How about:-
Maybe it is in the form of wire. It is superconducting so you can run current through it without destabilising it. You set up an arc in the reaction chamber between the wire and another electrode, or between two wires. The arc would break it down. The wire feed controls the supply of hydrogen to the chamber. The reaction will probably sustain itself, so the question is now "How do you stop it"? If you cant stop it the wire will continue to burn back through the chamber inlet seal and into the fuel wire store. Bang!
Last edited by elderflower (2016-11-06 11:38:31)
Offline
Like button can go here
#5 2016-11-07 10:39:24
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Interesting!
I wonder what the effect of introducing deuterons or alloying elements would be.
You could maybe make a pure fusion bomb have a metallice deuterium-tritium core surrounded by a mantle of metallic protonium-hydrogen. Ignite the protonium on all sides so you have an even burn. The metallic protonium turns to gaseous protonium producing inward compression of the core. While the outer shell is undergoing H + H combustion, the core is getting crushed, as it implodes it heats up, maybe to fusion temperatures, and basically what you have is a fusion bomb without plutonium!
Offline
Like button can go here
#6 2016-11-07 10:59:00
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
And could be made very small as its not limited by critical mass like a Plutonium initiated H bomb.
This could make the serial explosion propulsion system workable. There remains a problem with the half life of Tritium which would prevent it being used for long interstellar trips.
Offline
Like button can go here
#7 2016-11-07 12:59:19
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,325
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
If you query on alloys of metallic Hydrogen, using Lithium, you might find what you want. Lithium produces Tritium in nuclear reactions. And the alloy may become a solid at a much lower pressure. Yes, I feel the love.
Last edited by Void (2016-11-07 12:59:56)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#8 2016-11-07 16:39:34
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 4,000
- Website
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
I'm *sure* there was a story a while ago about hyperdense deuterium being mooted for fusion reactors, because the atoms were so close together it was much easier to trigger fusion.
Perhaps it would allow light, compact, deuterium fusion reactors for spacecraft, and proton fusion reactors for stationary applications (which can tolerate the massive reactor sizes required)? That would open up the Oort cloud to humanity. Including the fuel to slow down, and assuming a high efficiency engine, such ships could achieve velocities of ~4% of c with reasonable (3-4) mass ratios. That's plenty enough for island hopping across the stars, and the solar system proper would be a doddle.
Use what is abundant and build to last
Offline
Like button can go here
#9 2016-11-07 16:43:52
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 4,000
- Website
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
That's qraal, by the way.
Use what is abundant and build to last
Offline
Like button can go here
#10 2016-11-08 00:28:14
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
A Swedish physical chemist, Leif Homlid, is producing ultra-dense deuterium, though the news-bite is a bit vague on how much and how stable it is. However it’s 130,000 times denser than water and would make a fantastic fusion fuel in an ICR fusion system. Might even make deuterium rockets a whole lot easier than Friedwardt Winterberg has imagined. Sure would be a less bulky way of storing the stuff if it could be made in bulk.
130,000 times denser than water?!!! Metallic hydrogen is only 15 times as dense as liquid hydrogen, which makes it about as dense as water. Metallic deuterium would be twice as dense, but 130,000 times as dense? So one cubic meter of the stuff would weigh 130,000 tons? I wonder how much gravity it would have? That would be 130,000,000 kg. 867438000E -11 Newtons
Offline
Like button can go here
#11 2016-11-08 03:49:24
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Maybe somebody got his decimal point in the wrong place. That would be white dwarf stuff.
Offline
Like button can go here
#12 2021-02-15 03:23:49
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Similar topic; Does anyone know anything about the development of ultra dense deuterium?
https://www.cambridge.org/core/journals … 44781E1E33
Has this development gone anywhere in the past 10 years?
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#13 2021-02-19 14:45:50
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
More recent works suggest that metallic hydrogen remains metastable down to 20-10 GPa, and quickly decays in molecular hydrogen at lower pressure.
https://aip.scitation.org/doi/10.1063/1.5008406
I don't know it is possible to build a tank able to hold 20-10 GPa of pressure, but anyway it would be too massive for a rocket.
I'm very sad, because I also hoped to see a 1500 s Isp metallic hydrogen rocket.
Last edited by Quaoar (2021-02-20 04:40:12)
Offline
Like button can go here
#14 2021-02-20 04:40:23
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
More recent works suggest that metallic hydrogen remains metastable down to 20-10 GPa, and quickly decays in molecular hydrogen at lower pressure.
https://aip.scitation.org/doi/10.1063/1.5008406
I don't know it is possible to build a tank able to hold 20-10 GPa of pressure, but anyway it would be too massive for a rocket.
I'm very sad, because I also hoped to see a 1500 s Isp metallic hydrogen rocket.
Errata corrige:
On second thought, it would always be possible to trap metallic hydrogen into some sort of graphene cage (e.g. fullerene) to withstand 10-20 GPa of pressure
This kind of rocket would be similar to a classical LOX-LH2 rocket, but the LH2 is "doped" with a powder of fullerenes filled of metallic hydrogen (e.g. 25% in weight). Small amount of LOX is needed to burn the carbon cages and start the metallic hydrogen decay.
So imagine we have metallic hydrogen fullerenes and let's build the rocket:
chamber pressure 100 bar
area ratio 250
fuel: LH2 with 25% wt of metallic hydrogen
oxidizer : LOX
O/F ratio: 0.1
chamber temperature: 3337 K
vacuum exhaust velocity: 10.1 km/s (better than a solid core NTR but without the trouble related with nuclear)*
Simulation performed with NASA CEA
https://cearun.grc.nasa.gov/cgi-bin/CEARUN/donecea3.cgi
* Note: it is also possible to increment the exhaust velocity by augmenting the metallic hydrogen percentage, but it also rises the chamber temperature. Anyway, we can imagine some kind of vortex cooled chamber, where the reaction is confined in the meddle, which can withstand higher temperature. In this case we can have:
chamber pressure 100 bar
area ratio 250
fuel: LH2 with 50% wt of metallic hydrogen
oxidizer : LOX
O/F ratio: 0.1
chamber temperature: 4450 K
vacuum exhaust velocity: 13.7 km/s
Last edited by Quaoar (2021-02-20 08:02:25)
Offline
Like button can go here
#15 2021-02-20 08:05:51
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,104
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
On second thought, it would always be possible to trap metallic hydrogen into some sort of graphene cage (e.g. fullerene) to withstand 10-20 GPa of pressure
Thanks for following up with the graphene cage possibility!
I'd wondered if something like that might be possible,
(th)
Offline
Like button can go here
#16 2021-02-20 10:08:25
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Quaoar wrote:On second thought, it would always be possible to trap metallic hydrogen into some sort of graphene cage (e.g. fullerene) to withstand 10-20 GPa of pressure
Thanks for following up with the graphene cage possibility!
I'd wondered if something like that might be possible,
(th)
When I was a child, I visited with a school trip the central dairy of Rome and I was impressed seeing milk flowing into paper tubes which were transformed in tetra-pack by machines. I wonder if it may be possible to do the same with metastable metallic hydrogen: the diamond anvil synthesizes liquid MMH and makes it flow at high pressure into graphene tubes, which are cut and welded by lasers to form micrometric tetra-packs holding MMH.
Last edited by Quaoar (2021-02-20 10:16:53)
Offline
Like button can go here
#17 2021-02-20 10:20:39
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,104
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
For Quaoar re #16
It is a privilege for me to be able to offer encouragement for you to PLEASE continue your thinking!
Also, if you have not been aware, this forum is actively recruiting new "high value" members, and existing members are in the very best possible position to identify prospective members and to encourage them. You are the ONLY active member from Italy, so I hope you will keep a watch for candidates you'd like to see 'enrolled' in whatever process this forum is engaged in.
You've noted the creative thinking of Void, the expertise of GW Johnson and kbd512, and the Engineering capabilities of Calliban.
It is not only possible but likely I am failing to mention talents of many other existing members.
We have need of volunteers to help with projects by RobertDyck, Calliban, and (importantly to me) development of a Tutorial on Celestial Mechanics for Mission Planning that I hope will be led by GW Johnson.
We are holding a collection of work by GW Johnson that is packed with the knowledge he has accumulated over decades, and it is just sitting in archive waiting for development.
If you know of a professor who might be interested in helping to develop this material into a formal curriculum, please let that person know of the opportunity.
Clearly we need serious students to help to provide the incentive for volunteer work to build this tutorial. The candidates will see themselves as preparing to navigate manned and unmanned spacecraft between Earth and Mars (or anywhere in the Solar System).
If you would care to do so, you can become a lead for development of the Hydrogen storage method you've described. It may not be possible to engineer the storage system you've imagined, but since there is no way to know without actually trying, now is a good time to begin the effort.
(th)
Offline
Like button can go here
#18 2021-02-20 10:52:44
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,163
- Website
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
10 GPa = 98,692 standard atmospheres = 100,000 bar = 1,450,000 psi. There are no ways-and-means or materials to build a tank to hold pressures like that.
The smaller solid propellant rockets I worked with used chamber pressures in the 1000-5000 psi range. Big solids like the shuttle boosters used about 900 psi. No liquid or hybrid uses high tankage pressures, only the combustion chamber pressures are high: in the 300-4000 psi range.
There are waterjet cutting systems and diesel fuel injection systems that operate in the 10,000 to 50,000 psi range. They are very heavily built. Those are still two orders of magnitude short of 10 GPa.
I'm unsure how one might possibly contain something at pressures that high. There are diamond anvil research tools that reach "pressures" like that, but it is not containment, only applied force to a point.
GW
Last edited by GW Johnson (2021-02-20 10:54:53)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#19 2021-02-21 12:17:51
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Closed molecular cages would strike me as being the only option for storing something at a pressure of 10,000MPa. Something like this.
https://en.m.wikipedia.org/wiki/Buckminsterfullerene
You would need to force hydrogen into the fullerine molecules at extremely high pressures and relatively high temperatures. At sufficiently high temperatures, the bond length between the carbon atoms in the fullerine would be sufficient to make them porous to hydrogen molecules. What the critical temperature and pressure is, I don't know. Temperature would need to be low enough to prevent chemical reaction between the fullerine and hydrogen, but high enough to allow the fullerine to be porous to hydrogen molecules.
Next, you cool the fullerine to temperatures close to absolute zero. The molecular trap contracts, exerting enormous compressive forces on the trapped hydrogen. The hydrogen becomes metallic and remains trapped within the fullerine shell, as the bond length between the carbon atoms shrinks. Metallic hydrogen, maintained in lattice structure would also cease to display fluid properties, due to the atomic bonds between the H atoms.
One option might be to compress a mixture of gaseous hydrogen and fullerine using a hyper velocity impact using a light gas gun. The molecular fragments from the impact could then be quenched in super cooled helium gas.
Other options might be to diffuse hydrogen into perfect metal single crystals at high temperature and very high pressure and then cool the crystals to absolute zero. This would need to be done very quickly, as quenching the material would inevitably reduce the pressure in the surrounding hydrogen. Hydrogen trapped within the lattice will be compressed by the contraction of atomic bonds. Metallic hydrogen would form metallic bonds with other hydrogen atoms and would cease to be porous within crystal.
It may be difficult to find any substance strong enough to achieve this, even for perfect crystals. Monocrystaline silicon might be strong enough. Maybe diamond or ultra pure silica.
I can't imagine any of this being cheap. And even with an ISP of 1500 you would still need roughly a tonne of metallic hydrogen for each tonne of payload delivered to orbit. I doubt that it would be economically competitive, even if it is technically possible. If the molecular trap containing the metallic hydrogen can be used as fuel for an inertial confinement fusion engine, then the net energy return may be high enough to justify the likely enormous cost of each fuel pellet.
A couple of problems occur to me. So long as the hydrogen remains close to absolute zero, it is effectively trapped within the lattice of the crystal or fullerine molecule. However, in any material at apparently uniform temperature, there can be local variations in molecular energy. If even one fullerine molecule fails within your fuel pellet, the result may end up being a detonation wave. Secondly, such a concentrated source of energy, failing catastrophically, would produce a fireball hot enough to produce x-rays. I believe GW has written in the past about the EMP hazards of nuclear explosions in the upper atmosphere. That hazard occurs because fission generates gamma rays that ionise the upper atmosphere, creating electric field gradients which interact with powerlines. An intense x-ray source in the ionosphere could do the same thing.
All of this is conjecture on my part. So take it with a large heap of salt.
Last edited by Calliban (2021-02-21 13:31:21)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#20 2021-02-26 05:12:19
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
For Quaoar re #16
It is a privilege for me to be able to offer encouragement for you to PLEASE continue your thinking!
Also, if you have not been aware, this forum is actively recruiting new "high value" members, and existing members are in the very best possible position to identify prospective members and to encourage them. You are the ONLY active member from Italy, so I hope you will keep a watch for candidates you'd like to see 'enrolled' in whatever process this forum is engaged in.
You've noted the creative thinking of Void, the expertise of GW Johnson and kbd512, and the Engineering capabilities of Calliban.
It is not only possible but likely I am failing to mention talents of many other existing members.
We have need of volunteers to help with projects by RobertDyck, Calliban, and (importantly to me) development of a Tutorial on Celestial Mechanics for Mission Planning that I hope will be led by GW Johnson.
We are holding a collection of work by GW Johnson that is packed with the knowledge he has accumulated over decades, and it is just sitting in archive waiting for development.
If you know of a professor who might be interested in helping to develop this material into a formal curriculum, please let that person know of the opportunity.
Clearly we need serious students to help to provide the incentive for volunteer work to build this tutorial. The candidates will see themselves as preparing to navigate manned and unmanned spacecraft between Earth and Mars (or anywhere in the Solar System).
If you would care to do so, you can become a lead for development of the Hydrogen storage method you've described. It may not be possible to engineer the storage system you've imagined, but since there is no way to know without actually trying, now is a good time to begin the effort.
(th)
Thanks, I'm happy to collaborate, but I don't know if a SF writer like me, who likes hard fiction and dreams about interplanetary and interstellar spaceships, might be useful to a real project
Last edited by Quaoar (2021-02-27 04:31:29)
Offline
Like button can go here
#21 2021-02-26 05:20:15
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
A couple of problems occur to me. So long as the hydrogen remains close to absolute zero, it is effectively trapped within the lattice of the crystal or fullerine molecule. However, in any material at apparently uniform temperature, there can be local variations in molecular energy. If even one fullerine molecule fails within your fuel pellet, the result may end up being a detonation wave. Secondly, such a concentrated source of energy, failing catastrophically, would produce a fireball hot enough to produce x-rays. I believe GW has written in the past about the EMP hazards of nuclear explosions in the upper atmosphere. That hazard occurs because fission generates gamma rays that ionise the upper atmosphere, creating electric field gradients which interact with powerlines. An intense x-ray source in the ionosphere could do the same thing.
All of this is conjecture on my part. So take it with a large heap of salt.
That's not a big problem: MMI fullerenes need in any case to be diluted with liquid hydrogen to lower the chamber temperature, so you can use them to obtain a gelled liquid hydrogen and store it at 10 K with a zero-boil-off active cooling system.
Last edited by Quaoar (2021-02-26 05:27:15)
Offline
Like button can go here
#22 2021-02-26 06:48:51
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,104
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
For Quaoar re #21 and #20
Nice response to the concern of Calliban! I am hoping he will follow up! Keeping a large quantity of energy dense matter at 10 degrees K seems like the kind of thing Scotty in Startrek (tm) would have found routine, although the science fiction writers of the day did not mention it. They were dealing in "magic" at the time, and you and Calliban are looking to see if there might be some science under the magic.
Re #20 ....
Thanks, I'm happy to collaborate, but I don't know if a SF writer like me, who likes hard fiction and dreams about interplanetary and interstellar spaceship, might be useful to a real project
The need (as I see it) is to recruit the kind of talented and highly motivated person to assist with forum projects in a disciplined manner.
The people who will be settling Mars, and helping in thousands of ways to make it possible through labors on Earth, are alive on Earth today. To my knowledge, NONE of these people are members of this forum, although there is certainly a possibility that CaptJTorriani may be one of them.
We have open (volunteer) opportunities for a master chef to assist with meal planning for the Large Ship, several kinds of engineers to assist with design of the Large Ship, and numerous opportunities for qualified individuals to assist Calliban with numerous ideas.
A particularly attractive project under discussion here is Ballistic Delivery of supplies to Mars. This should be of interest to anyone with a military orientation, although in this case, instead of destroying objects with ballistics, we are looking to preserve the contents of the delivery packages so they can be used on Mars without a lot of recovery effort.
(th)
Offline
Like button can go here
#23 2021-02-28 11:31:58
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,516
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
GW is correct. There's no way to contain such pressures using known composite materials, to include graphene and CNT composites. Any suitable storage vessel would undoubtedly be so heavy as to negate any density impulse advantage achieved by using metallic hydrogen. The diamond anvils capable of creating such extreme pressures are enormous. If you need a 1,500s Isp from Hydrogen propellant, then a liquid core nuclear reactor can achieve that without invoking pressure vessel technology that doesn't exist.
Offline
Like button can go here
#24 2023-03-15 08:08:11
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
NASA had delays with its rocket launch to Moon with liquid hydrogen, not an easy for for Heavy Lift and it needs attention.
There are always new designs and new studies ideas, what to use on the Moon, could a railgun work, what to build when you get to Mars for using methane, biofuel, Fluorine-Hydrogen, Fluorine-Ammonia, LH2/LOX, Ozone-Hydrogen, Fluorine-Diborane save fuel with an Ion drive, more power is needed in space so let's use Nuclear. Metallic hydrogen has a very high predicted Isp, it is also predicted that if hydrogen and deuterium have liquid metallic states, they might have quantum ordered states that cannot be classified as superconducting or superfluid in the usual sense. Instead, they might represent two possible novel types of quantum fluids: superconducting superfluids and metallic superfluids.
'The most detailed analysis of metallic hydrogen as rocket propellant I've ever seen'
https://twitter.com/ToughSf/status/1611709238815985665
Most exciting advances, opportunities might one day come.
Superconductivity physical property
More superconductor news, high temperature and relative low pressure?
Scientists discover superconducting material that could bring total revolution in energy and electronics
https://news.yahoo.com/scientists-disco … 28993.html
The new material is described in a paper, ‘Evidence of near-ambient superconductivity in a N-doped lutetium hydride’, published in Nature today.
The material has been nicknamed “reddmatter”, after its colour and as a nod to a material from Star Trek. It found that name during the process of creating it, when scientists found that it surprisingly switched to become a “very bright red” while it was being created.
Professor Dias and the team made the material by taking a rare earth metal named lutetium and mixed it with hydrogen and a small part of nitrogen
Offline
Like button can go here
#25 2024-05-17 08:02:26
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Co-discover of Metallic Hydrogen wrote paper on metallic hydrogen for
Stunning Images From Juno’s Close Flyby of Jupiter Unveil Hidden Surprise
https://scitechdaily.com/stunning-image … -surprise/
From LaH10 to room–temperature superconductors
https://www.nature.com/articles/s41598-020-58065-9
Pressure-induced hydride superconductors above 200 K
https://pubs.aip.org/aip/mre/article/6/ … -above-200
the material with the highest accepted superconducting temperature was highly pressurized lanthanum decahydride, whose transition temperature is approximately 250 K (−23 °C) at 200 GPa
What is Metallic Hydrogen?
https://www.allthescience.org/what-is-m … drogen.htm
Although the metallic hydrogen produced at the Lawrence Livermore National Laboratory was solid, it has been theorized that it may be possible to create a liquid version, if even greater pressures, around 4 million atmospheres, are used. Calculations have also determined that this material might be a superconductor at room temperature, although this property would be somewhat useless for practical purposes, as the cost of compressing something to over a million atmospheres for an extended period of time is much greater than cooling something down to near absolute zero. However, there is a small chance that metastable metallic hydrogen might be possible -- that is, one that retains its phase even when the pressure is removed.
1998 article
High-Pressure Scientists 'Journey' To The Center Of The Earth, But Can't Find Elusive Metallic Hydrogen
https://www.sciencedaily.com/releases/1 … 080541.htm
Metallic Hydrogen - Potentially a High Energy Rocket Propellant
https://ntrs.nasa.gov/citations/20080018926
Pure metallic hydrogen is predicted to have a specific impulse (Isp) of 1700 seconds, but the reaction temperature is too high for current engine materials. Diluting metallic hydrogen with liquid hydrogen can reduce the reaction temperature to levels compatible with current material limits and still provide an Isp greater than 900 s. Metallic hydrogen has not yet been produced on earth, but experimental techniques exist that may change this situation.
Offline
Like button can go here