New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2015-12-02 13:32:21
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,347
A process line on Mars based on sand dunes and ice and sunlight.
How about this?
http://earthsky.org/space/amazing-new-i … ov-25-2015
Before I start my predatory stalking behaviors towards this sand dune and it's kin, I might remind the viewers, that to my knowledge it is not alive, and that in the universe there must be countless Mars like worlds with unpredated sandunes. ![]()
I would have put this information in another topic, but, I needed one that will not be sliced to pieces for not being "On Topic", because to make a profit from such a mineral deposit, you try to get as much as you can from it, only letting the squeak go. Actually I have more sympathy for pigs than you might think. I think it is the first farm animal we should phase out, because of it's reported intelligence, not for religious reasons. The danger of cancer is another reason not to eat animals. But please comment a rebuttal elsewhere. This dune to my knowledge is non-living, and does not feel pain. I do want to get the most out of it however.
Next;
http://space.io9.com/sand-dunes-are-rai … 1678443555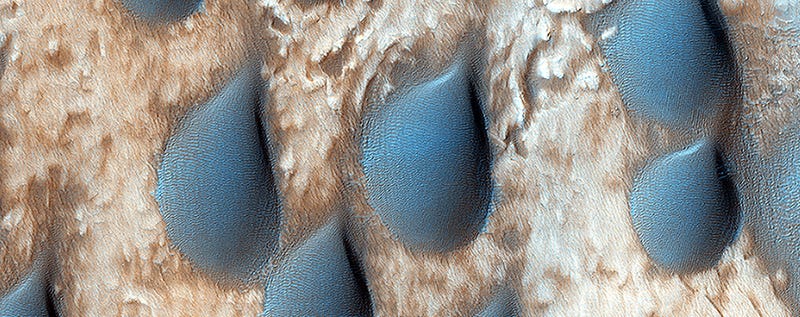
It's raining dunes! Well, no, not really, but these olivine-rich sand dunes on Mars really do look like classic cartoon drawings of raindrops sliding across the landscape.
Next;
I hope to digest the dune material by the use of;
https://en.wikipedia.org/wiki/Serpentinite
Formation and petrology[edit]
Serpentinization is a geological low-temperature metamorphic process involving heat and water in which low-silica mafic and ultramafic rocks are oxidized (anaerobic oxidation of Fe2+ by the protons of water leading to the formation of H2) and hydrolyzed with water into serpentinite. Peridotite, including dunite, at and near the seafloor and in mountain belts is converted to serpentine, brucite, magnetite, and other minerals — some rare, such as awaruite (Ni3Fe), and even native iron. In the process large amounts of water are absorbed into the rock increasing the volume and destroying the structure.[1]
The density changes from 3.3 to 2.7 g/cm3 with a concurrent volume increase on the order of 30-40%. The reaction is highly exothermic and rock temperatures can be raised by about 260 °C (500 °F),[1] providing an energy source for formation of non-volcanic hydrothermal vents. The magnetite-forming chemical reactions produce hydrogen gas under anaerobic conditions prevailing deep in the mantle, far from the Earth's atmosphere. Carbonates and sulfates are subsequently reduced by hydrogen and form methane and hydrogen sulfide. The hydrogen, methane, and hydrogen sulfide provide energy sources for deep sea chemotroph microorganisms.[1]
Serpentinite reactions[edit]
So;
I am thinking the mixing of dune materials and superheated steam might get us the favor of "H2", and oddly Clay for bricks.
So where could you get superheated steam to accelerate this process?
(Solar power towers)
Got any premade excavated locations for that sort of thing?
https://en.wikipedia.org/wiki/Impact_crater
(For when you might really size this thing up, if you feel you need to.)
Now at some point we might want to figure out if we could get some metals out of the dunes before we turn them into H2 and Clay/Bricks.
Along with other works elsewhere it's been discussed here.
http://newmars.com/forums/viewtopic.php?id=7316
We should at least hope to get some Chromium, Iron, and Titanium as well as some Basalt fibers/cloth out of the dune before we finalize the process.
OK, so we have some H2, and it appears almost everywhere, Martian Atmosphere.
Previous discussions indicate that we can burn H2 in Martian atmosphere. It requires a high temperature, but it can be done.
However, I prefer a fuel cell. More efficient I presume, and perhaps can be done at pressures closer to Martian ambient pressure.
But Antius and others want to do stuff with Liquid CO2. Why not indulge them (If it works).
How about atmospheric separations.
We might hope to separate the atmosphere of Mars into a liquid CO2 component, and "The Remnant".
http://newmars.com/forums/viewtopic.php?id=7150
Antius and his kind get to play with the liquid CO2. Otherwise can play with "The Remnant".
Which is likely to include N2, Argon, O2, and CO.
(You might also try reverse Osmosis to get the CO2 out).
So;
You have this H2 which was derived in a non-biological way. You also have the atmospheric remnant gas.
And Fuel Cells. I presume that the O2 and CO can act against H2 as Oxidizers.
That's electricity then from the fuel cells perhaps, and a further remnant gas, N2 and Argon, and the production of H2O and CH4 (Methane).
Do what you like with that. Release to atmosphere for a greenhouse effect, or use Cryrogenics to get what you want out of it.
You let the squeak go, since it is non-existant, I don't think sand dunes squeak.
I think I might have done pretty good, but I a bit tipped just now. I might have to do some clean up later, and some amendments.
Last edited by Void (2015-12-02 14:28:22)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#2 2016-02-03 18:03:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: A process line on Mars based on sand dunes and ice and sunlight.
Offline
Like button can go here
