New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2013-05-31 12:00:18
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Ice Worms
Ice worms were first discovered in 1887 on Muir Glacier in southeast Alaska by George Frederick Wright, a glacial geologist (who doubled as a theologian) in 1887. However, the discovery was more or less ignored for most of the 20th century. Only very recently has any real effort been made to subject them to thoroughgoing scientific enquiry.
The coastal glaciers that serve as ice worm habitat extend from Mount Rainier in Washington State to Alaska.
Ice worms, which are a relative of the common earthworm, have no eyes, so they don't see images, but they respond to light and dark. At a little less than an inch long (one to two centimeters), they are often described as looking like pieces of dark thread in the ice.
They have big mouths, said Shain, and their primary food source is the red algae that grows on glaciers. Their ideal temperature is zero degrees Celsius (32 degrees Fahrenheit); at 5 degrees Fahrenheit they start to disintegrate.
They spend their days burrowing up and down through the ice. Biologist Dan Shain remarks that "They definitely operate on some kind of Circadian rhythm, moving up when it's dark and down when it's light."
Ice worms propel themselves using setae, extremely small bristles that protrude from the sides of their bodies. Wriggling through fractured ice and snow crystals, they burrow as deep as three to six feet (one to two meters) beneath the surface of the ice. They have mysterious head pores which might be producing some sort of lubricant to help them move through the ice.
They can live in colonies of a few hundred thousand to 20 million, covering an area as large as 30 acres. No one knows how long they live. Beyond the occasional bird or two, the only threat to their existence may be global warming.
Although temperatures on Mars are probably too cold for our cosseted Earth-bound ice worms, one can imagine that something like an ice worm might be able to live out life in the huge Martian glaciers. If so, then once again it is unlikely that our presence will threaten them. It is probably a case of ourselves having to exercise a degree of caution in choosing our water sources and in filtering and sterilising the water.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#2 2013-12-17 09:09:52
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Ice Worms
Ice worms were first discovered in 1887 on Muir Glacier in southeast Alaska by George Frederick Wright, a glacial geologist (who doubled as a theologian) in 1887. However, the discovery was more or less ignored for most of the 20th century. Only very recently has any real effort been made to subject them to thoroughgoing scientific enquiry.
The coastal glaciers that serve as ice worm habitat extend from Mount Rainier in Washington State to Alaska.
Ice worms, which are a relative of the common earthworm, have no eyes, so they don't see images, but they respond to light and dark. At a little less than an inch long (one to two centimeters), they are often described as looking like pieces of dark thread in the ice.
They have big mouths, said Shain, and their primary food source is the red algae that grows on glaciers. Their ideal temperature is zero degrees Celsius (32 degrees Fahrenheit); at 5 degrees Fahrenheit they start to disintegrate.
They spend their days burrowing up and down through the ice. Biologist Dan Shain remarks that "They definitely operate on some kind of Circadian rhythm, moving up when it's dark and down when it's light."
Ice worms propel themselves using setae, extremely small bristles that protrude from the sides of their bodies. Wriggling through fractured ice and snow crystals, they burrow as deep as three to six feet (one to two meters) beneath the surface of the ice. They have mysterious head pores which might be producing some sort of lubricant to help them move through the ice.
They can live in colonies of a few hundred thousand to 20 million, covering an area as large as 30 acres. No one knows how long they live. Beyond the occasional bird or two, the only threat to their existence may be global warming.
Although temperatures on Mars are probably too cold for our cosseted Earth-bound ice worms, one can imagine that something like an ice worm might be able to live out life in the huge Martian glaciers. If so, then once again it is unlikely that our presence will threaten them. It is probably a case of ourselves having to exercise a degree of caution in choosing our water sources and in filtering and sterilising the water.
Mars Express has found an ice lake http://www.space.com/1371-ice-lake-mars.html
It may be intriguing to hypothize some kind of martian ice worms, that breath a little amounth of oxigen produced via radiolysis or are anaerobian.
Last edited by Quaoar (2013-12-17 09:11:15)
Offline
Like button can go here
#4 2013-12-17 19:28:45
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
Well, it's not impossible but it seems unlikely-- more the realm of science fiction than science fact, so to speak.
That seems an absurd comment to me. We know organisms can exist in water and can metabolise ferrous oxide.
Why wouldn't creatures be able to exist in a benign ice environment?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#5 2013-12-17 19:40:54
Re: Ice Worms
Several reasons:
Mars likely did not have a warm-wet environment for long enough for complex life, such as an ice worm, to develop
Cells require liquid water to function. On Mars, this means massive amounts of salt that would necessarily inhibit the enzymes and vital biological functions of a cell, not even to mention a more complex, megamulticellular organism
Metabolize Ferrous Oxide--- excuse me?
If life could survive in this kind of environment, one would naturally expect it to propagate planetwide in a very visible manner through evolution. This is especially true if megamulticellular organisms can survive, because monocellular organisms are much hardier. We have never discovered any evidence that this is the case, therefore it is unlikely that this is a locale that supports life
Mars' water deposits are probably both highly acidic and strong oxidizers, both of which present significant threats and impediments to the survival of life forms within
If there were multicellular organisms on Mars, there would be all sorts of other kinds of organisms. The presence of organisms necessarily implies high levels (not the ppb range that may or may not have even been detected) of Methane. Because methane is certainly lower than .01 ppm planetwide (in fact, Curiosity results have just come out putting a lower bound to atmospheric composition around 2 ppb, which is to say that they haven't detected any at all), there are presumably not lifeforms.
Impossible? No. Likely or even reasonable? Not so much. That's why this is perfect fodder for science fiction.
-Josh
Offline
Like button can go here
#6 2013-12-18 15:07:54
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,349
Re: Ice Worms
Quote:
•Cells require liquid water to function. On Mars, this means massive amounts of salt that would necessarily inhibit the enzymes and vital biological functions of a cell, not even to mention a more complex, megamulticellular organism
I almost entirely agree with you but on this point, water ice can be liquid without salt. Exposed ice can serve as a greenhouse window, and articles I have read indicate that this is widly true. Don't know how acid the ice and it's melt water would be.
With that favor I pretty much agree with you. The lack of Methane is a killer.
Further, it is hard to see how tiny plants and worms could migrate from one isolated pocket of water to another.
I recall that this was put forward however as a means for panspermia from Earth to Mars. An Earth rock impacting a temperate ice field could expose clear ice, be embeded in it and for a time period have melted water around it. So entering the Martian undergrounds.
No evidence of it though.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#7 2013-12-18 15:15:47
Re: Ice Worms
While that's not entirely false, it also misses the pressure issue. In Mars gravity, each meter of water ice createsa pressure of 3.7 kPa, where Earth's surface pressure is 100 kPa. You would need at least a meter or two of ice to generate the pressure to create a significant liquid range for water. With this much ice, I would expect sunlight to be absorbed or reflected prior to reaching levels where water could be pressurized. Therefore, for this effect to be meaningful you still need lots and lots of salt.
The thing about life spreading is that, no matter how unlikely, it happens. Everywhere on Earth, no matter how isolated or inimical, has life, usually complex multicellular life. If there is life anywhere on the planet, it will be everywhere.
-Josh
Offline
Like button can go here
#8 2013-12-18 16:22:34
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,349
Re: Ice Worms
Well, here I finally found something related to what I was referencing. It seems that some persons believe that a rock falling into an ice field, can create a pocket of water, in this case while it does not hurt to have a water column, for instance on Mars, I estimate that 1 foot of water is worth about
10 mb pressurization. The water in question if fresh would be 0 degrees C, and not much pressurized above the Martian ambient, and in fact in some locations, that air pressure approaches 12 mb at times?
But, I think they may also speculate on a bottling effect, where the ice holds temporary pressuization during high noon by it's strength, and a thin film of liquid water may form around or even inside of a rock.
http://mpainesyd.com/filechute/paine_am … permia.pdf
One is where solar radiation heats
subsurface ice which melts and the pocket of melt-water retains sufficient pressure to
avoid sublimation. This could be quite common where ice is present and is exposed to
partial sunlight.
3 Finding
Using Antarctica as an analogy, it is estimated that 12% of the ice on Mars would
be exposed to sufficient sunlight to melt sub-surface ice in summer each year
(speculative). Assuming three months of sufficient sunlight per year then, on average,
2% of all of the surface ice could be expected to have liquid water near the surface.
This means that, on average, 0.015% (2% x 0.73%) of the Mars surface has liquid
water near the surface.
This is another interesting article I encounted during the search. It points out that the Mars climate is variable, as to pressure and tilt (Therefore location of snowfall) and that could also make ice melts more likely during variations.
http://www.planetary.org/blogs/emily-la … hesis.html
But we agree that the Methane is missing.
And please note, none of the above orignates from me (Obiously), so, I am mearly parroting things I read, no innovation being attempted here.
Last edited by Void (2013-12-18 16:26:59)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#9 2013-12-18 16:49:49
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
Several reasons:
Mars likely did not have a warm-wet environment for long enough for complex life, such as an ice worm, to develop
Cells require liquid water to function. On Mars, this means massive amounts of salt that would necessarily inhibit the enzymes and vital biological functions of a cell, not even to mention a more complex, megamulticellular organism
Metabolize Ferrous Oxide--- excuse me?
If life could survive in this kind of environment, one would naturally expect it to propagate planetwide in a very visible manner through evolution. This is especially true if megamulticellular organisms can survive, because monocellular organisms are much hardier. We have never discovered any evidence that this is the case, therefore it is unlikely that this is a locale that supports life
Mars' water deposits are probably both highly acidic and strong oxidizers, both of which present significant threats and impediments to the survival of life forms within
If there were multicellular organisms on Mars, there would be all sorts of other kinds of organisms. The presence of organisms necessarily implies high levels (not the ppb range that may or may not have even been detected) of Methane. Because methane is certainly lower than .01 ppm planetwide (in fact, Curiosity results have just come out putting a lower bound to atmospheric composition around 2 ppb, which is to say that they haven't detected any at all), there are presumably not lifeforms.
Impossible? No. Likely or even reasonable? Not so much. That's why this is perfect fodder for science fiction.
I wouldn't say it was likely but -
Mars life could have been seeded from Earth via meteorite impact.
Cells require water, but that does not mean that a multicellular creature has to have the same salt content as surrounding water/ice.
From Wikipedia:
"Ferrous iron (Fe2+
) oxidation[edit]For further information, see Acidophiles in acid mine drainage
Ferrous iron is a soluble form of iron that is stable at extremely low pHs or under anaerobic conditions. Under aerobic, moderate pH conditions ferrous iron is oxidized spontaneously to the ferric (Fe3+
) form and is hydrolyzed abiotically to insoluble ferric hydroxide (Fe(OH)
3). There are three distinct types of ferrous iron-oxidizing microbes. The first are acidophiles, such as the bacteria Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans, as well as the archaeon Ferroplasma. These microbes oxidize iron in environments that have a very low pH and are important in acid mine drainage. The second type of microbes oxidize ferrous iron at cirum-neutral pH. These micro-organisms (for example Gallionella ferruginea, Leptothrix ochracea, or Mariprofundus ferrooxydans) live at the oxic-anoxic interfaces and are microaerophiles. The third type of iron-oxidizing microbes are anaerobic photosynthetic bacteria such as Rhodopseudomonas,[7] which use ferrous iron to produce NADH for autotrophic carbon dioxide fixation. Biochemically, aerobic iron oxidation is a very energetically poor process which therefore requires large amounts of iron to be oxidized by the enzyme rusticyanin to facilitate the formation of proton motive force. Like sulfur oxidation, reverse electron flow must be used to form the NADH used for carbon dioxide fixation via the Calvin cycle."
The pattern might be that there was planet-wide life of this type but that it has retreated to small colonies as conditions have deteriorated. Is it not possible that Mars life forms might not
produce methane in the quantities you suggest. It doesn't look to me like the above microbes produce methane.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#10 2013-12-18 17:17:12
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Ice Worms
I very much doubt that multicellular life could have evolved on Mars. There wasn't enough time before it salted-up/acidified/went dry/went cold. Only about 1 B yrs was available before it all went south. Evolving multicellular life apparently took almost 3 B yrs here.
That being said, I would be very surprised that single-celled life never happened on Mars. In fact, I would bet it's still there, underground.
From what I read, there was some sort of microbe that helped precipitate the iron out of the ocean here as the banded-iron formations, around 2 B years ago. Combination of geology, chemistry, and biology. Although, being an engineer not a biologist or a scientist, I might have misunderstood what I read.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#11 2013-12-18 22:19:37
Re: Ice Worms
Void- The thing about Earth vs. Mars is low pressure. If that ice has liquid water underneath it, it will be at high pressure and be likely to crack. It would be tough for an ice layer thick enough to exert significant pressure to be transparent enough for its presence to result in a significant warming effect.
Beyond that, my general contention is that it's very tough for there to be an "in between" for life. Conditions are either so inimical that it never developed, or it will be everywhere. We see it on Earth. Life fills every niche, every hollow, every hole, stream, rock, and log. What we see is that evolution is an immensely powerful force that will drive any system subject to it to expand wildly in a geological eyeblink. If it's there on Mars, it would have done the same; or it would have died.
-Josh
Offline
Like button can go here
#12 2013-12-19 09:01:14
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,349
Re: Ice Worms
I will resort to lichen as the example life form that could benefit,
and agree that ice worms would require even more favor than the lichen.
Lichens have been shown to be able to survive and even like Mars like conditions in cracks in rocks, getting water,
from the dawn and dusk dews, not even needing water in ice.
But some Lichens like to grow inside of rocks in Antarctica.
Is there a condensation process that can lay clear or translucent water ice over sandstone or other rock that
lichen could grown in?
Perhaps in some climatic locations on Mars???
If so then I suggest that it can bottle pressure inside wet bubbles in the ice (Until they rupture).
This could be a repetitive watering process where ice is deposited, the sun comes up and heats the rock through
the ice, and a short period of wet occurs on the rock surface where pressurization is maintained by the ice
deposited on the rock, and then very likely a rupture. But such a period of wetness might be all that is needed.
I do not expect life in assocation with CO2 Ice eruptions, but it's model can suggest a similar one for water ice
on a less noticible scale.
Much like the dry bubbles created with clear CO2 ice as mentioned here:
CO2 (The only reason you can see the spider-Trees is that these ruptured).
http://en.wikipedia.org/wiki/Martian_geyser
From the above reference (Strangely), an interesting idea, I suppose water column or container pressurization are possible.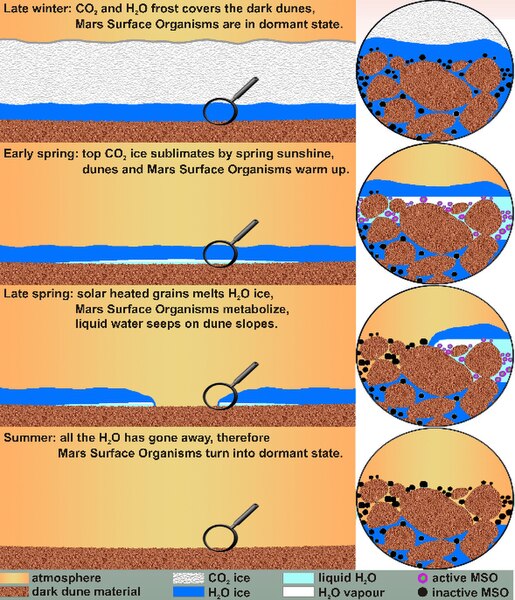
But this is all speculation.
I think the point is that it is very likely that there are temporary improvements of certain locations on Mars to the degree that it could favor life
more than normal. How that life could be established in those locations? Spores in the wind?
Last edited by Void (2013-12-19 09:09:13)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#13 2013-12-19 18:25:44
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
Void- The thing about Earth vs. Mars is low pressure. If that ice has liquid water underneath it, it will be at high pressure and be likely to crack. It would be tough for an ice layer thick enough to exert significant pressure to be transparent enough for its presence to result in a significant warming effect.
Beyond that, my general contention is that it's very tough for there to be an "in between" for life. Conditions are either so inimical that it never developed, or it will be everywhere. We see it on Earth. Life fills every niche, every hollow, every hole, stream, rock, and log. What we see is that evolution is an immensely powerful force that will drive any system subject to it to expand wildly in a geological eyeblink. If it's there on Mars, it would have done the same; or it would have died.
You didn't seem to know that microbes could metabolise ferrous oxide:
"Metabolize Ferrous Oxide--- excuse me?"
I am not sure I trust your judgment on what might or might not be possible.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#14 2013-12-19 18:37:09
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
I agree - we don't know enough about Mars yet to be able to say there are no benign environments. We know from Earth that whole ecosystems can exist in tiny spaces e.g. caves miles below the surface.
I will resort to lichen as the example life form that could benefit,
and agree that ice worms would require even more favor than the lichen.Lichens have been shown to be able to survive and even like Mars like conditions in cracks in rocks, getting water,
from the dawn and dusk dews, not even needing water in ice.But some Lichens like to grow inside of rocks in Antarctica.
Is there a condensation process that can lay clear or translucent water ice over sandstone or other rock that
lichen could grown in?Perhaps in some climatic locations on Mars???
If so then I suggest that it can bottle pressure inside wet bubbles in the ice (Until they rupture).
This could be a repetitive watering process where ice is deposited, the sun comes up and heats the rock through
the ice, and a short period of wet occurs on the rock surface where pressurization is maintained by the ice
deposited on the rock, and then very likely a rupture. But such a period of wetness might be all that is needed.I do not expect life in assocation with CO2 Ice eruptions, but it's model can suggest a similar one for water ice
on a less noticible scale.
Much like the dry bubbles created with clear CO2 ice as mentioned here:
CO2 (The only reason you can see the spider-Trees is that these ruptured).
http://en.wikipedia.org/wiki/Martian_geyserFrom the above reference (Strangely), an interesting idea, I suppose water column or container pressurization are possible.
http://upload.wikimedia.org/wikipedia/e … DS_MSO.jpgBut this is all speculation.
I think the point is that it is very likely that there are temporary improvements of certain locations on Mars to the degree that it could favor life
more than normal. How that life could be established in those locations? Spores in the wind?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#15 2013-12-19 19:54:58
Re: Ice Worms
In what is perhaps an ironic twist of fate, Mars has almost no ferrous oxide. The highly oxidizing environment has reduced all of the Iron to the Ferric (Iron (III)) state, leaving little or no Ferrous (Fe (II)) Iron compounds.
My outrage was therefore both correct and justified; Would you care to make any further assertions about my ability to opine correctly on the matter?
I don't doubt that it's physically possible for life to exist on Mars, but rather make an argument similar to that which leads to the Fermi Paradox on a Galactic scale. "If they were there, they would be everywhere because on geological timescales, life spreads quickly. We don't see any life. So where is it?"
There's nothing unreasonable, in the case of Mars, in saying that it's not there.
-Josh
Offline
Like button can go here
#16 2013-12-20 11:25:24
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Ice Worms
Nobody will know until somebody looks inside rocks from way more than a meter underground, with something way better than a magnifying lens. Here on Earth, there are single-cell creatures that live in the pores of deep rocks. That kind of life may well outweigh all of the life on the surface, no one yet knows. If our surface environment were to "die" the way Mars's evidently did, those underground microbes would still be there.
GW
edit 12-21-13 to "single-cell" from "single-"
Last edited by GW Johnson (2013-12-21 10:28:20)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#18 2013-12-21 07:58:48
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
In what is perhaps an ironic twist of fate, Mars has almost no ferrous oxide. The highly oxidizing environment has reduced all of the Iron to the Ferric (Iron (III)) state, leaving little or no Ferrous (Fe (II)) Iron compounds.
My outrage was therefore both correct and justified; Would you care to make any further assertions about my ability to opine correctly on the matter?
I don't doubt that it's physically possible for life to exist on Mars, but rather make an argument similar to that which leads to the Fermi Paradox on a Galactic scale. "If they were there, they would be everywhere because on geological timescales, life spreads quickly. We don't see any life. So where is it?"
There's nothing unreasonable, in the case of Mars, in saying that it's not there.
Well you have to explain why there are so many references to Mars being rich in iron oxide:
http://www.universetoday.com/22580/why-is-mars-red/
What's your source for saying there is little or no iron oxide?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#19 2013-12-21 08:50:41
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 4,000
- Website
Re: Ice Worms
Iron Oxide =/= Ferrous Oxide. What Mars has is *Ferric* Oxide; Fe2O3 rather than FeO.
Use what is abundant and build to last
Offline
Like button can go here
#20 2013-12-21 10:36:09
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Ice Worms
To answer Josh's question in post #17 above: the evolutionary pressure to re-expand from underground onto the surface always exists. The surface conditions may (or may not) be too harsh to permit it. On Mars, it appears that surface conditions may currently be too harsh. I do not know whether the radiation, the chemistry, or the near-vacuum air pressure (that forces water to vaporize away) is the worst, but the combination of those three is rather daunting for life on the surface.
As for the form-of-iron discussion: life finds a way to utilize what is there, as long as too-harsh conditions don't prevent it. Underground on Mars is not as harsh as the surface. Buried ice does not sublime, liquid water might even be stable given some heat. Radiation is far reduced, down to nil the deeper you go. Subsurface chemistry is different from surface chemistry here, so probably there as well; it's just that we won't know how it differs until we go there and drill deep.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#21 2013-12-22 00:07:06
Re: Ice Worms
GW: I don't doubt that the surface is harsh. But I presume that we agree that it is not impossibly so. Given that, and given that there is a definite continuum from a meter or two below the surface to open air I would certainly expect the increasing availability of energy resources and new niches to fill would present a strong evolutionary pressure for life to migrate up.
Louis, Terraformer got it exactly right. I'd add that a strong knowledge of chemistry is quite important if you're trying to convince someone that... what are the words again? Oh yes, if you want me and others to be "sure I trust your judgment on what might or might not be possible" when it comes to exobiology.
In any case, there would be no need to metabolize any form of Iron oxide when there are sufficient reserves of water and carbon dioxide lying around.
-Josh
Offline
Like button can go here
#22 2013-12-22 17:14:52
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ice Worms
GW: I don't doubt that the surface is harsh. But I presume that we agree that it is not impossibly so. Given that, and given that there is a definite continuum from a meter or two below the surface to open air I would certainly expect the increasing availability of energy resources and new niches to fill would present a strong evolutionary pressure for life to migrate up.
Louis, Terraformer got it exactly right. I'd add that a strong knowledge of chemistry is quite important if you're trying to convince someone that... what are the words again? Oh yes, if you want me and others to be "sure I trust your judgment on what might or might not be possible" when it comes to exobiology.
In any case, there would be no need to metabolize any form of Iron oxide when there are sufficient reserves of water and carbon dioxide lying around.
Why do microbes on earth metabolise iron oxide then?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#23 2013-12-22 18:16:14
Re: Ice Worms
Ferrous iron is a soluble form of iron that is stable at extremely low pHs or under anaerobic conditions. Under aerobic, moderate pH conditions ferrous iron is oxidized spontaneously to the ferric (Fe3+) form and is hydrolyzed abiotically to insoluble ferric hydroxide (Fe(OH)3). There are three distinct types of ferrous iron-oxidizing microbes. The first are acidophiles, such as the bacteria Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans, as well as the archaeon Ferroplasma. These microbes oxidize iron in environments that have a very low pH and are important in acid mine drainage. The second type of microbes oxidize ferrous iron at cirum-neutral pH. These micro-organisms (for example Gallionella ferruginea, Leptothrix ochracea, or Mariprofundus ferrooxydans) live at the oxic-anoxic interfaces and are microaerophiles. The third type of iron-oxidizing microbes are anaerobic photosynthetic bacteria such as Rhodopseudomonas, which use ferrous iron to produce NADH for autotrophic carbon dioxide fixation. Biochemically, aerobic iron oxidation is a very energetically poor process which therefore requires large amounts of iron to be oxidized by the enzyme rusticyanin to facilitate the formation of proton motive force. Like sulfur oxidation, reverse electron flow must be used to form the NADH used for carbon dioxide fixation via the Calvin cycle.
That is to say, Iron is metabolized by a few rather rare forms of bacteria, most often to eliminate an excess it from their environment. It doesn't seem to necessarily be a primary source of energy for these organisms, either.
By the way, if we're talking chemistry, it only makes sense to oxidize FeO in an environment where oxygen is common, because relative to oxidizing sugars it releases much less energy per oxygen atom. This is not the case on Mars, where the atmosphere contains a measly 1 Pa of Oxygen. Compare: Earth's atmosphere has 21,000 Pa of Oxygen.
-Josh
Offline
Like button can go here
#24 2013-12-23 06:55:42
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,349
Re: Ice Worms
I have nothing to add on Iron. (Don't know).
But, I have been considering why life from Earth may not be surviving long term on Mars. For instance my favorite traveler now would be a piece of sandstone from Earth that would have organisms that live a quarter of an inch or so inside the rock. Maybe capable of photosynthisis.
I have thought that if such a rock landed on sandstone where there was a regular depositing of water ice, in the darkness, and then a warming in the day, there is the potential
of morning and sunset dews (Frosts). Lichen can absorb mosisture directly from unmelted frost or snow. Plus, it is possible for liquid water to exist long enough in each thaw for lichen (Or other life) to get a drink. Then there is the possibility of a glaze of ice melting from the bottom up as mentioned in a previous post.
So I have wondered why Mars may not host such organisms. Josh said the methane if missing, and I actually buy into that. However, if the amount of life was very small, perhaps it could be there.
It seems that there could be small habitats that get watered even now, and it appears that when Mars has a greater tilt of axis, the Equator may be both watered, and warm enough for daily melts, cold enough for nightly snows and frosts.
That seems the best situation for life to me.
But what is Mars like if it has a Zero degree axis? (If it ever does).
http://en.wikipedia.org/wiki/Axial_tilt
Mars' obliquity is currently in a chaotic state; it varies as much as 0° to 60° over some millions of years, depending on perturbations of the planets.[16][26] The obliquities of the outer planets are considered relatively stable. Some authors dispute that Mars' obliquity is chaotic, and show that tidal dissipation and viscous core-mantle coupling are adequate for it to have reached a fully damped state, similar to Mercury and Venus.[2][27]
I am thinking that the water gets locked up in the poles, and does not experience enough warmth for thawing. So the high lattitudes are watered (Ice) but cold, and the lower lattitudes are arid but warm.
This could be very unfavorable to surface life, if it persists for a very long time.
And as Josh has suggested the aquifers below may be very salty and unfavorable to life as well.
So, this could be a sterilization process.
Perhaps Earth life was established on the surface of Mars (Or more likely a fraction of an inch inside of sandstone) several times, only to be made extinct during such an unfavorable event as lack of axis tilt.
Aside from that I have wondered if at zero degrees axis it might be possible that the CO2 component of the atmosphere could collapse into the poles, dropping the air pressure even more than now. Making Mars even more aggressively hostile to life. Don't know about that. Maybe. It would be for the same reason the Moon is thought to have water ice in shaded spots at the poles.
Last edited by Void (2013-12-23 07:00:51)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#25 2013-12-23 15:12:23
Re: Ice Worms
Void:
It is my claim that there is no such thing as limited amounts of life. Once it exists anywhere, it will either adapt or evolve to fill all niches. Take the example of... Us. 300,000 years ago, there were no humans on the planet. Once we evolved, we spread quite quickly out of the Great Rift Valley into other locales where we could not previously have thrived or even survived. In doing so, we often found our biology changing. For example, our skin became lighter the farther north we traveled; In Asia our eyes became narrower and developed folds to keep out dust as we traveled through deserts. In various locales we became taller or fatter. At the onset of the Ice Age, we were pushed to our limit but survived, and as it ended human civilization began. What we see is that in time we thrived in places where we could previously only barely survive.
This process is characteristic of all forms of life; And if we are to assume that life on Mars did not come to be in the last ten million years (quite a reasonable supposition, yes) we would have expected that it would either have died or learned to thrive. That means that if it's there it's on the surface in a visible way; That means it's far from the equator. That means that when we look for organic compounds in the soil, we find them.
This is not the case. Ergo, if life is there it's in the process of dying (If life was transplanted or initiated on Mars in the last ten million years chances are it happened many times previously) or it was never there at all.
-Josh
Offline
Like button can go here