New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2020-11-30 18:36:36
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,345
New tech can get oxygen, fuel from Mars's salty water
------
I could not let this pass without notice:
https://phys.org/news/2020-11-tech-oxyg … salty.html
Quote:
New tech can get oxygen, fuel from Mars's salty water
When it comes to water and Mars, there's good news and not-so-good news. The good news: there's water on Mars! The not-so-good news?
There's water on Mars.
The Red Planet is very cold; water that isn't frozen is almost certainly full of salt from the Martian soil, which lowers its freezing temperature.
You can't drink salty water, and the usual method using electricity (electrolysis) to break it down into oxygen (to breathe) and hydrogen (for fuel) requires removing the salt; a cumbersome, costly endeavor in a harsh, dangerous environment.
If oxygen and hydrogen could be directly coerced out of briny water, however, that brine electrolysis process would be much less complicated—and less expensive.
Engineers at the McKelvey School of Engineering at Washington University in St. Louis have developed a system that does just that. Their research was published today in the Proceedings of the National Academy of Sciences (PNAS).
The research team, led by Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished University Professor in the Department of Energy, Environmental & Chemical Engineering, didn't simply validate its brine electrolysis system under typical terrestrial conditions; the system was examined in a simulated Martian atmosphere at -33 F (-36 C).
"Our Martian brine electrolyzer radically changes the logistical calculus of missions to Mars and beyond" said Ramani. "This technology is equally useful on Earth where it opens up the oceans as a viable oxygen and fuel source"
In the summer of 2008, NASA's Phoenix Mars Lander "touched and tasted" Martian water, vapors from melted ice dug up by the lander. Since then, the European Space Agency's Mars Express has discovered several underground ponds of water which remain in a liquid state thanks to the presence of magnesium perchlorate—salt.
In order to live—even temporarily—on Mars, not to mention to return to Earth, astronauts will need to manufacture some of the necessities, including water and fuel, on the Red Planet. NASA's Perseverance rover is en-route to Mars now, carrying instruments that will use high-temperature electrolysis. However, the Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) will be producing oxygen only, from the carbon dioxide in the air.
The system developed in Ramani's lab can produce 25 times more oxygen than MOXIE using the same amount of power. It also produces hydrogen, which could be used to fuel astronauts' trip home.
"Our novel brine electrolyzer incorporates a lead ruthenate pyrochlore anode developed by our team in conjunction with a platinum on carbon cathode" Ramani said. "These carefully designed components coupled with the optimal use of traditional electrochemical engineering principles has yielded this high performance."
The careful design and unique anode allow the system to function without the need for heating or purifying the water source.
"Paradoxically, the dissolved perchlorate in the water, so-called impurities, actually help in an environment like that of Mars," said Shrihari Sankarasubramanian, a research scientist in Ramani's group and joint first author of the paper.
"They prevent the water from freezing," he said, "and also improve the performance of the electrolyzer system by lowering the electrical resistance."
Typically, water electrolyzers use highly purified, deionized water, which adds to the cost of the system. A system that can work with "sub-optimal" or salty water, such as the technology demonstrated by Ramani's team, can significantly enhance the economic value proposition of water electrolyzers everywhere—even right here on planet Earth.
"Having demonstrated these electrolyzers under demanding Martian conditions, we intend to also deploy them under much milder conditions on Earth to utilize brackish or salt water feeds to produce hydrogen and oxygen, for example through seawater electrolysis," said Pralay Gayen, a postdoctoral research associate in Ramani's group and also a joint first author on this study.
Such applications could be useful in the defense realm, creating oxygen on demand in submarines, for example. It could also provide oxygen as we explore uncharted environments closer to home, in the deep sea.
The underlying technologies enabling the brine electrolyzer system are the subject of patent filing through the Office of Technology Management and are available for licensing from the university.
If this it what it seems on face value, then perhaps it is massively important for Mars and for Space travel.
It is believed that there is a brine water table on Mars, and it is typically easier to work with fluids than it is to work with solid ice.
Done.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#2 2020-11-30 19:24:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,129
Re: New tech can get oxygen, fuel from Mars's salty water
For Void re new topic !!! Congratulations on this impressive find!
The topic ** should ** build with entries as members follow and report on developments.
I'm glad to see (a) the technology is in process for patent, and (b) that it will be licenses!
The patent means the process technology (IP) will be visible and available to study.
The license opportunity means anyone with the means and the expertise to build a company ** should ** be able to start development of affordable implementations for the global marketplace.
The detail about the suspended matter on Mars ** helping ** the reaction was a nice surprise!
Again, ** thanks ** for this encouraging find.
(th)
Offline
Like button can go here
#3 2020-11-30 19:43:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: New tech can get oxygen, fuel from Mars's salty water
I am wondering if a current could be passed through the soil in a container that would allow for the electrolyzed gasses to be captured from the selected soil. We know that plates are used in a normal tank so would blade plates that cut into the soil aid in surface area to pass that current through the soils with.
The container would have loose soils within it until the blades are pushed into it where it would then be compressed to allow for more contact to be achieved. So compression would also force the water free as well.
Depending on location from the rovers only a couple cups of water are present in the soils but to what depth and does the water content increase with that depth.
Offline
Like button can go here
#4 2020-12-10 20:20:05
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: New tech can get oxygen, fuel from Mars's salty water
Water on Mars not as widespread as previously thought, study finds
Researchers combined data on brine evaporation rates, collected through experiments at the center's Mars simulation chamber, with a global weather circulation model of the planet to create planetwide maps of where brines are most likely to be found.
Researchers considered more variables and took global weather patterns into account to create maps of where water might be found.
Favorable conditions for stable brines on the planet's surface are most likely to be present in mid- to high-northern latitudes, and in large impact craters in the southern hemisphere, he said.In the shallow subsurface, brines might be present near the equator.
In the best-case scenario, brines could be present for up to 12 hours per day. "Nowhere is any brine stable for an entire day on Mars," he said.
We will need to drill to prove it out...
Offline
Like button can go here
#5 2022-05-05 10:43:17
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: New tech can get oxygen, fuel from Mars's salty water
Solar Beats Nuclear At Many Potential Settlement Sites On Mars
http://spaceref.com/mars/solar-beats-nu … -mars.html
China plans to invite other nations and even commercial partners to work and stay on its Tiangong space station
https://www.dailymail.co.uk/sciencetech … ation.html
Lunar soil has the potential to generate oxygen and fuel
https://phys.org/news/2022-05-lunar-soi … -fuel.html
Moon soil can turn carbon dioxide into oxygen, study finds
https://www.independent.co.uk/climate-c … 72138.html
Offline
Like button can go here
#6 2024-01-19 14:18:40
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,129
Re: New tech can get oxygen, fuel from Mars's salty water
The article at the link below is dated January 17, 2024
https://interestingengineering.com/inno … lever-tech
An integrated plant that will remove 50,000 tonnes of carbon dioxide every year and create new freshwater from salty seawater is planned in the Daesan Industrial Complex in South Korea. When ready, this will be the world's first such facility.
As countries work on their promises to go carbon neutral in a few decades, there is a strong push for innovative approaches that capture and utilize carbon. Carbon capture facilities work onsite to help reduce the release of carbon into the atmosphere. In contrast, direct air capture (DAC) technology focuses on removing the released carbon dioxide from the atmosphere.
Typically, these technologies are deployed alone with their singular goal of removing carbon and need supporting infrastructure for efficient working. Interesting Engineering has previously reported how direct air capture technology has turned water-positive and created fresh water as a byproduct. Capture6, another DAC technology company, is using it to achieve more than just capturing CO2.
South Korea's water and carbon woes
The Daesan Industrial Complex is responsible for 40 percent of South Korea's petrochemical production. However, droughts over the past several years have resulted in severe water scarcity in the region and dependence on external water sources.
K-water, the state-owned water utility company, is building a desalination plant to meet its water needs. However, being a hub of petrochemical production, the area generates 17MtCO2e of greenhouse emissions yearly.
Capture6's water-positive DAC technology offers an opportunity to address both these issues at once, the first of its kind in the world.
'World first': South Korea to tackle CO2 & saltwater with clever techIllustration on how Capture6's technology is water positive
Capture6How does the technology work?
The integrated plant to be set up in the industrial area will feature Capture6's Project Octopus, which will collaborate with K-water's desalination facility. The saltwater from the facility will be used to create a carbon removal solvent for its direct air capture applications.
"This collaboration is a major step forward for Capture6," said Ethan Cohen-Cole, co-founder and CEO of Capture6. "By pioneering water integrated water management and CO₂ removal facility, this project will significantly contribute to the region's sustainable future."
As per the company's press release, this approach will help provide an affordable method for carbon removal while also increasing freshwater yields. Capture6 also intends to address concerns about the environmental harms of the desalination plant.
Conventionally, the desalination process results in the creation of brine, which harms marine ecosystems when disposed of in the sea or ocean. Capture6 plans to use the brine to make green chemicals like hydrochloric acid and calcium carbonates, which are needed for industrial applications in South Korea. Locally, the green chemicals will help decarbonize K-water's water management operations.
These chemicals are made using fossil fuels and imported to South Korea. By making them locally, with waste from another process, Capture6 will help reduce the environmental impact of the region's industrial activities and replace them with a circular economy, making it more sustainable than before.
"As a public institution, we are committed to leading the global carbon neutrality efforts and nurturing the domestic water industry by partnering with the private sector to develop innovative water technologies,” added Yun Seog Dae, CEO of K-water.
(th)
Offline
Like button can go here
#7 2024-01-20 08:24:14
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: New tech can get oxygen, fuel from Mars's salty water
another article that directs the efforts to Suck carbon out of the atmosphere and desalinate seawater at the same time? This startup is trying.
If it’s ultimately able to grow into a commercial-scale facility, Project Octopus could capture up to 500,000 metric tons of CO2 annually once fully completed. The pilot facility is supposed to draw down just 500 metric tons of atmospheric carbon dioxide a year, and the MOU also includes plans to filter another 500 metric tons of CO2 from smokestacks before it’s released into the atmosphere.
Altogether, that’s just a fraction of the 17 million metric tons of carbon dioxide Daesan pumps out each year. And to reach global goals of stopping climate change set under the Paris Agreement, polluters need to slash their CO2 emissions in half this decade. The plan at Project Octopus is to start construction on the $2-3 million pilot this year, but breaking ground on a potentially $100-200 million commercial facility might not happen until late 2026 at the earliest.
Offline
Like button can go here
#8 2024-01-22 18:58:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: New tech can get oxygen, fuel from Mars's salty water
Here is another method
Researchers unlock energy-efficient solution to global water crisis
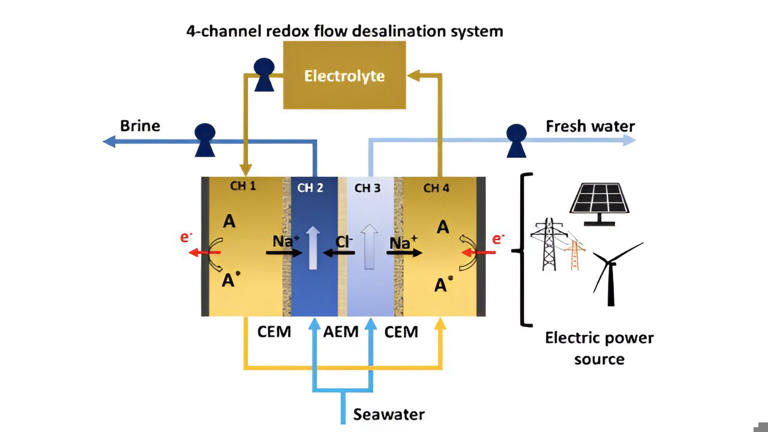
The intricacies of the system involve the division of incoming seawater into two streams: The salinating stream (see image above, CH 2) and the desalinating stream (image above, CH 3). Two additional channels house the electrolyte and redox molecule (image above, A). These channels are effectively separated by either a cation exchange membrane (CEM) or an anion exchange membrane (AEM).
In CH 4, electrons are supplied from the cathode to the redox molecule, extracting Na+ that diffuses from CH 3. The redox molecule and Na+ are then transported to CH 4, where electrons are supplied to the anode from the redox molecules, and Na+ is allowed to diffuse into CH 2. Under this overall potential, Cl- ions move from CH 3 through the AEM to CH 2, forming the concentrated brine stream. Consequently, CH 3 generates the freshwater stream.
Researchers at NYU Tandon School of Engineering achieved a major breakthrough in Redox Flow Desalination (RFD), an emerging electrochemical technique that can turn seawater into potable drinking water and also store affordable renewable energy.
In a paper published in Cell Reports Physical Science, the NYU Tandon team led by Dr. André Taylor, professor of chemical and biomolecular engineering and director of DC-MUSE (Decarbonizing Chemical Manufacturing Using Sustainable Electrification), increased the RFD system's salt removal rate by approximately 20% while lowering its energy demand by optimizing fluid flow rates.
Offline
Like button can go here