New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2020-09-09 16:20:49
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Liquid Co2 reactor cooling
An abundant supply of dry ice makes possible the construction of extremely simple nuclear power reactors. At temperatures beneath 150C, we could construct graphite moderated reactors, using aluminium pressure tubes running through a lightly pressurised graphite stack. At such low temperatures, aluminium magnesium alloys experience negligible creep. The fuel could be aluminium clad, natural uranium metal slugs or balls within the pressure tubes. No enrichment required. The CO2 would be injected as a liquid and would convert to supercritical fluid as it passes through the core. A direct cycle should be possible, with supercritical CO2 passing directly from the core into a compact single stage turbine. We could even tap CO2 off at about 10 bar into a main that supplies the base power tools.
This is a nuclear reactor that could be built on Mars using very basic materials and presumably, entirely domestic resources, provided that we can find a suitable source of natural uranium on Mars. The CO2 exhaust would be ducted through a set of ovens, which would be loaded with dry ice. Once full, the oven will be sealed and CO2 exhaust ducted through it. The liquefied CO2 would then be injected into the reactor using centrifugal pump.
Last edited by Calliban (2020-09-09 16:29:15)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#2 2020-09-09 17:52:53
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For Calliban re #29
SearchTerm:DryIceFissionReactor
SearchTerm:NuclearPowerDryIce
(th)
Offline
Like button can go here
#3 2020-09-09 21:05:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
The loading of dry ice into the chambers could be automated so as to keep man out of the way of the return lines extreme heat that would be at the oven valves and chamber area.
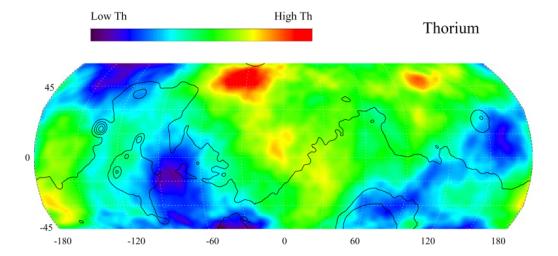
While I know thorium is plentiful is there enough Uranium to make the rods or pebbles on mars?
The orbiter called "2001 Mars Odyssey" mapped several Mars resources. One was thorium. The reason is thorium is an indicator mineral for uranium; meaning go here to do further prospecting.
However, I believe we can use thorium itself. On Earth, there's 3 times as much thorium as uranium, and 100% of thorium in nature can be used as reactor fuel, while only 0.72% of uranium is U-235. The issue was Canada has Earth's largest deposits of uranium, and the United States has the second. There's more uranium here than thorium. And you can't make a bomb from thorium, while you can from uranium. The fact a thorium reactor is much safer, and it's almost impossible to use it for weapons manufacturer, is one reason it should be developed. But US military is not interested for exactly those reasons. India developed a thorium reactor to power a small town. One reason is they have much more thorium than uranium; I think they have the largest thorium deposit on Earth. So why not just use thorium itself?
100% of thorium in nature is ²³²Th. That's the correct way to write it. The only superscripts built into the standard font are '2' and '3', so I don't have to write it as Thorium-232.
Offline
Like button can go here
#4 2020-09-09 21:25:35
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For either Calliban or SpaceNut .... could you clarify what the temperatures are going to be for this system?
"Extreme heat" seems (to me at least) like an unlikely scenario. As I recall, the forum contains at least one post that shows the melting point of dry ice.
My impression is that the system would never see temperatures higher than the freezing point of water, if they got that high.
(th)
Offline
Like button can go here
#5 2020-09-09 21:53:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
The out put of the boiler pan where the Uranium is steam that is super heated and why we need it to be clear of all minerals and as such mars will be operating in a simular mode was as the co2 will be gaseous and heated into the super critical range of the image in post 27. Sure wished that the temperature was not in kelvin as to get the everyday interpretation of it we need to convert to Celsius or to Fahrenheit degrees....
Offline
Like button can go here
#6 2020-09-10 01:53:07
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Liquid Co2 reactor cooling
The CO2 would become liquid at a temperature of 220K (-53C) and a pressure of 5.1bar. At this temperature, any contaminating water ice should remain frozen. So the oven need not reach high temperatures. A bigger problem would be nitrogen-16, which is an intense gamma emitter resulting from neutron irradiation of oxygen in the core of the reactor. It has a half life of 7 seconds. In boiling water reactors, the turbine is shielded to protect operators from unnecessary dose. To protect operators against this hazard in the external ovens, we could use automation; a secondary heat exchanger; or we could design the oven such that material drops down a long chute. The flue from the turbine would likely be exhausted up a tall stack. As for CO2 powering hand tools and other mechanical equipment, we would need arrangements (I.e a long pipe) ensuring that at least several half lifes had passed before CO2 arrives at the point of use.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#7 2020-09-10 06:49:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For Calliban re #34
Thanks for helping forum readers to understand more about your vision of an application of fission to deliver useful outputs on Mars.
For SpaceNut ... would you be willing to confirm that Calliban's design does NOT produce the kind of heat you are used to seeing on Earth.
This Mars oriented design appears (to me at least) to be a radically difference way of looking at the challenge. If I understand the proposal correctly, at NO point does ANY component of the reactor exceed the temperature of boiling water.
Calliban, because SpaceNut appears to be "seeing" high temperatures in his mental model of your design, can you spend a bit more time on the issue of temperature. Fissionable materials are going to produce heat. Are you planning to pull heat out of the reactor at such a rate that the temperature of the working fluid does NOT exceed that of boiling water?
***
Regarding charging of CO2 for power tools ... Is it reasonable to consider charging vessels designed for storage of CO2 under pressure, and letting them sit in "cooling" areas until they can be moved to the work site safely?
(th)
Offline
Like button can go here
#8 2020-09-10 16:40:39
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Liquid Co2 reactor cooling
For Calliban re #34
Thanks for helping forum readers to understand more about your vision of an application of fission to deliver useful outputs on Mars.
For SpaceNut ... would you be willing to confirm that Calliban's design does NOT produce the kind of heat you are used to seeing on Earth.
This Mars oriented design appears (to me at least) to be a radically difference way of looking at the challenge. If I understand the proposal correctly, at NO point does ANY component of the reactor exceed the temperature of boiling water.
Calliban, because SpaceNut appears to be "seeing" high temperatures in his mental model of your design, can you spend a bit more time on the issue of temperature. Fissionable materials are going to produce heat. Are you planning to pull heat out of the reactor at such a rate that the temperature of the working fluid does NOT exceed that of boiling water?
***
Regarding charging of CO2 for power tools ... Is it reasonable to consider charging vessels designed for storage of CO2 under pressure, and letting them sit in "cooling" areas until they can be moved to the work site safely?(th)
The higher the outlet temperature of the reactor, the greater the efficiency. However, outlet temperatures could be less than 100C. Because the pressure tubes are made of aluminium alloy (which is cheap, abundant and easy to make on Mars) the temperature of the coolant cannot be greater than 150C, as aluminium has a limited creep life at temperatures greater than that. So for this design, you would probably want operating temperature to be no higher than the boiling point of water.
I have assumed the use of solid uranium metal fuel which is clad in aluminium, mainly because it is cheap and easy to make, has low neutron absorption cross section and operating temperatures are low enough to allow it. Gaseous fission products will tend to pressurise the cladding, so we wouldn't want fuel surface temperature to get much above 150C, as it would then be vulnerable to bursting. If fuel operates at 150C surface temperature, a 100C coolant temperature allows reasonable rates of heat transfer into the CO2. A reactor outlet temperature greater than 60C would be desirable if we intend to make use of process heat for things like hot water and habitat heating.
In principle, we could charge up steel cylinders for later use. No doubt this will be done in situations where portable power is needed. Piping room temperature CO2 into workshops is another option. We could do this after we had extracted excess heat for habitat heating by running the steel pipes through water tanks.
It is doubtful that it would be possible for coolant temperatures to safely exceed 100C with aluminium fuel cladding in an aluminium pressure tube reactor. However, much higher operating temperatures could be achieved using a pebble bed reactor. In this case, fuel would be in the form of solid graphite balls, with uranium dioxide particles embedded within them. Liquid CO2 would be injected into a waste heat recovery boiler before entering the reactor. Operating temperatures must be greater than 250C, in order to avoid buildup of wigner energy. However, operating much above 400C would be difficult, due to CO2 reaction with graphite and doppler broadening of 238U absorption spectrum, which makes it more difficult to sustain a critical reaction in natural uranium at higher temperatures.
The liquid CO2, natural uranium, graphite moderated, aluminium pressure tube reactor, is a good first generation design because it is very easy to build. But it must be built close to a source of dry ice, so is really limited to polar regions. However, the design has a lot of strengths that may make this a worthwhile compromise. As noted already, the reactor is simple and cheap to build. As a pressure tube reactor, it also has the advantage of being easily scalable to extremely high power levels. If you want a reactor with ten times as much power, you just make the unpressurised moderator stack 3 times wider with ten times more pressure tubes. No need for a thick pressure vessel. With natural uranium fuel, there are no bottlenecks associated with enrichment either. A design like this could be used to build extremely powerful nuclear reactors, quickly and cheaply on Mars. Exactly what is needed for a planet on which lots of cheap energy is a survival necessity.
A pebble bed reactor could presumably be built anywhere on Mars. But it would be more expensive to build because it needs a pressure vessel.
Last edited by Calliban (2020-09-10 17:11:20)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#9 2020-09-10 17:12:15
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling

Seems that we will not have an liquid co2 to cool the rods and the water content will need to build up to make it a steam generator with the water.
If we vent each gas from dry ice thats dumped on the reactor and generate power from each we are at a very high pressure for each.
Offline
Like button can go here
#10 2020-09-10 17:48:35
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Liquid Co2 reactor cooling
If we are talking about a boiling CO2 reactor, then maximum operating temperature is 31C, as that is the critical point of CO2. Operating at higher temperatures means using supercritical coolant. The main problem here is that density drops from ~750kg/m3 for liquid CO2 at 300K, to just 325kg/m3 for supercritical CO2 at 310K. Both at pressure 8MPa. Check out the NIST fluid properties database.
https://webbook.nist.gov/chemistry/fluid/
Boiling allows much better rates of heat transfer as it involves phase change, which absorbs a lot of energy. So a boiling CO2 reactor would probably work at or around room temperature. The pressure tubes would never get hotter than 30C.
Last edited by Calliban (2020-09-10 17:49:58)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#11 2020-09-12 08:43:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
The reactor would need processed quality controlled co2 to be the feed stock so we will need a dry ice purification system and process to save what we mine for the other gasses that would be present in what we dig up.
We can use a solar, or coolant loop from a reactor to aid in giving the heat source for that purification process in a separate low pressure chamber where danger and safety are control for a human coal burning like shoveling it in as the door seal must be clean of debris in order to properly close after each filling. The controlled temperature of the chamber would allow for selective gas retrieval and refinement for later use. Those could be that buffer gasses we need for farming to habitat when it comes to nitrogen. For argon we would want that for ion ship building as well. With the surplus co2 not needed for the reactor being used for pressure powered tools.
Offline
Like button can go here
#12 2020-09-12 09:02:12
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For SpaceNut re #39
I am looking forward to seeing Calliban's reply about the dry ice to be fed into his reactor design.
It is a mystery to me why any action would be needed to prepare the dry ice for input to the nuclear reactor "boiler". Dry ice on Mars should be as pure as nature can make a material like that. What are you thinking would be present that could in any way impact the operation of the reactor?
(th)
Offline
Like button can go here
#13 2020-09-12 09:09:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
That would only be dry ice created on a cooling plate that RobertDyck has designed for mars society. name escapes me currently
My post 7 does a combined approach as you use the cold plate at night turning it off each morning so as to save on energy to get the co2 without scraping the dry ice off from the plates.
Mined dry ice would not be pure and would require processing to do so.
Update
found the post in this topic http://newmars.com/forums/viewtopic.php?id=8815
my post 12/13 is about cleaning the co2 gas from mined co2. RobertDyck has a post with the name just after that....
Mars Atmospheric Carbon Dioxide Freezer in post 20
Another thing is air. Pioneer Astronautics developed the Mars Atmosphere Carbon DiOxide Freezer (MACDOF). It uses the principle that Mars at night is almost cold enough to freeze CO2 into dry ice. A fan blows Mars air through a filter to remove dust (fines) into a canister with freezer coils. CO2 freezes as dry ice frost. When it's full, seal the canister and warm the dry ice to sublimate it into CO2 gas. That will pressurize the canister so you don't need any pump at all. Ingenious design, but doesn't collect anything other than CO2.
That's enough to produce fuel for an ERV, but a settlement needs other things. That can be modified by pumping filtered Mars air into the canister, pressurizing to 10 bars, then freeze dry ice. All other gasses will accumulate, including CO. Since slightly more than 95% of Mars air is CO2, that will concentrate everything else 20 times, including CO. That would bring CO to a toxic level. Add a catalyst like the catalytic converter from a car to combine CO + O2 -> CO2. There isn't much O2 in Mars air, but there's more of it than CO. The catalyst will also break ozone into O2, not necessary but it will do so. Actually, NASA developed a catalyst for breathing masks when evacuating a burning building that does this at 20°C, a lot cooler than exhaust from a car. The catalyst at the top of the chamber would be heated to +20°C while freezer coils at the bottom are cooled to 170°K (-103.15°C). Both in one canister will take power, but it'll remove all CO. As CO2 is frozen it'll reduce pressure, a sensor will control a pump to add more Mars air, maintaining 10 bar pressure. Once the pressure doesn't drop any further, close a valve and turn off the pump, but keep the catalyst warm and coils cold. Then when CO levels drop below detection threshold by a sensor, turn off the catalyst heater but keep the coils cold. A thermometer will monitor temperature, when it stops getting colder you're done. Then open a second valve to a "mix gas" holding tank and use a fast scavenger pump to capture as much as possible, reducing canister pressure and pressurizing the storage tank. When that's done close the valve, and open a third valve to a CO2 holding tank. Then heat the dry ice to sublimate it. It only takes -78.5°C to sublimate dry ice so that can use low level waste heat from manufacturing processes or low pressure steam from the reactor's secondary coolant. You don't need to pump out CO2, as it sublimates it'll pressurize itself. There's a trace of water in Mars air, which will freeze as ice in -103.15°C and the -78.5°C won't melt it, so the canister will accumulate a little ice. There isn't much, but it'll have to be melted out a couple times a year.
Offline
Like button can go here
#14 2020-09-12 10:15:37
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For SpaceNut re #41
Thanks for clarifying all the similar and somewhat related subtopics that came to mind when Calliban proposed his (to me innovative) reactor design.
As far as I can tell (subject to correction of course) Calliban's design does NOT require any preparation of the dry ice minded from natural collections.
The model that I think fits best is the good old-fashioned coal furnace, which I had the privilege of learning how to operate. Raw coal is full of contaminants, and no processing ahead of combustion is performed, other than crushing so the pieces can be shoveled conveniently. The combustion process releases gases and leaves solids behind, which (in the vernacular of the region where I lived) were called clinkers. That could be because they made a nice musical sound under the right circumstances.
In any case, as I understand Calliban's design, the raw dry ice would be fed into the "boiler" of the fission reactor, where the CO2 would be released to a gaseous form, and any solids that might be present would fall (under Mars gravity) to the floor of the "boiler".
It might be necessary to scrape the bottom of the "boiler" now and then, but I would expect the volume of such debris to be quite small.
I would be interesting to know if the debris collected has a musical tone. I rather doubt it, because Calliban's design barely breaks the temperature of boiling water (although I'm not sure what temperature water boils on Mars, which has such a tenuous atmosphere).
A quick check with Google revealed this:
But on Mars, where the atmosphere is much thinner than on Earth, it can boil at temperatures as low as 0 °C. During the Martian summer, when the subsurface water ice begins to melt and emerge at the surface, where the mean temperature reaches 20 °C, it immediately starts to boil.May 2, 2016
Although boiling, water does shape Martian terrain ...
It remains for Calliban to clarify if he meant boiling water on Earth, or on Mars.
In any case, your concerns about hot systems appear to have led to discovery of a remarkable invention, for an environment that will be available to humans at Mars distance and beyond.
Edit#1: Elderflower posted this helpful advice: http://newmars.com/forums/viewtopic.php … 12#p147012
The difference between now and when Louis first created this topic is that Calliban has introduced an innovated fission reactor design to provide the heating.
Obviously Calliban's design is far more reliable, ** and ** it is usable in any part of the Solar system where dry ice is abundant and solar energy is in short supply.
(th)
Last edited by tahanson43206 (2020-09-12 10:22:42)
Offline
Like button can go here
#15 2020-09-12 11:00:19
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
Water for mars locked up in dry ice is going to be salty with perchlorates and more so with the mined areas as more dust dirt will be mixed in. The water would be full of contaminants once the co boils off in the chamber and that is why you would not put the dry ice into the reactor boiler directly. We also would want the dry ice to form a liquid co2 for use in the boiler section of the reactor loop with the steam outlet going to the power turbines and then on exit into the cooling loops which will be used to preheat the dry ice mining or collection boiler process to allow for clean co2 to be made useable. There is no gas co2 around the reactors rods in use as you need fluid contact to pull that heat away with in the form of steam.
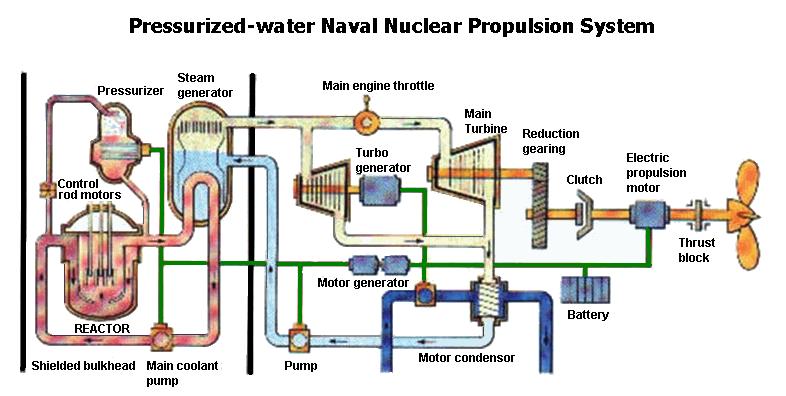
Difference is with liquid co2 in the loop
Offline
Like button can go here
#16 2020-09-12 12:39:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For SpaceNut re #43
It is helpful if we (NewMars contributors) can all use the same terminology.
Would you be willing to look up the definition of "dry ice" and update your post #43 to reflect whatever you learn?
(th)
Offline
Like button can go here
#17 2020-09-12 13:49:09
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
clean dry ice anywhere on mars....no.... as DRY ICE on earth is manufactured from clean co2
https://www.nasa.gov/sites/default/file … elease.pdf
“Mining” Water Ice on Mars An Assessment of ISRU Options in Support of Future Human Missions
Offline
Like button can go here
#18 2020-09-12 14:27:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For SpaceNut re #45
Calliban is ** NOT ** talking about water ice on Mars.
He ** is ** talking about frozen Carbon Dioxide on Mars.
Carbon Dioxide precipitated out of the atmosphere would be pure as the "driven snow" to coin an Earthly term.
Why would you think it is dirty?
And! Why would it matter for Calliban's reactor?
The "clinkers" (if there are any) would be scraped out of the boiler once a month or so.
The duties of NewMars forum Administrator are very demanding, and I understand that is necessary to switch between very different topics from one moment to the next.
Furthermore, I understand that the term "dry ice" is misleading, and it probably should not have been used, but at the time it probably seemed like a reasonable way to describe the substance to a population which was very poorly educated.
Edit#1: Here is the person (apparently) responsible for creating the confusing term "dry ice"
Dry ice - Wikipedia
https://en.wikipedia.org/wiki/Dry_ice
OverviewHistoryPropertiesManufactureApplicationsExtraterrestrial occurrenceSafetyBibliography
It is generally accepted that dry ice was first observed in 1835 by French inventor Adrien-Jean-Pierre Thilorier (1790–1844), who published the first account of the substance. In his experiments, it was noted that when opening the lid of a large cylinder containing liquid carbon dioxide, most of the liquid carbon dioxide quickly evaporated. This left only solid dry ice in the container. In 1924, Thomas B. Slate applied for a US patentto sell dry ice commercially. Subsequently, he became th…
Wikipedia · Text under CC-BY-SA license
(th)
Last edited by tahanson43206 (2020-09-12 14:39:33)
Offline
Like button can go here
#19 2020-09-12 16:30:34
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
Mars atmosphere is full of dust as well as for a mixture of gasses at very low pressure.
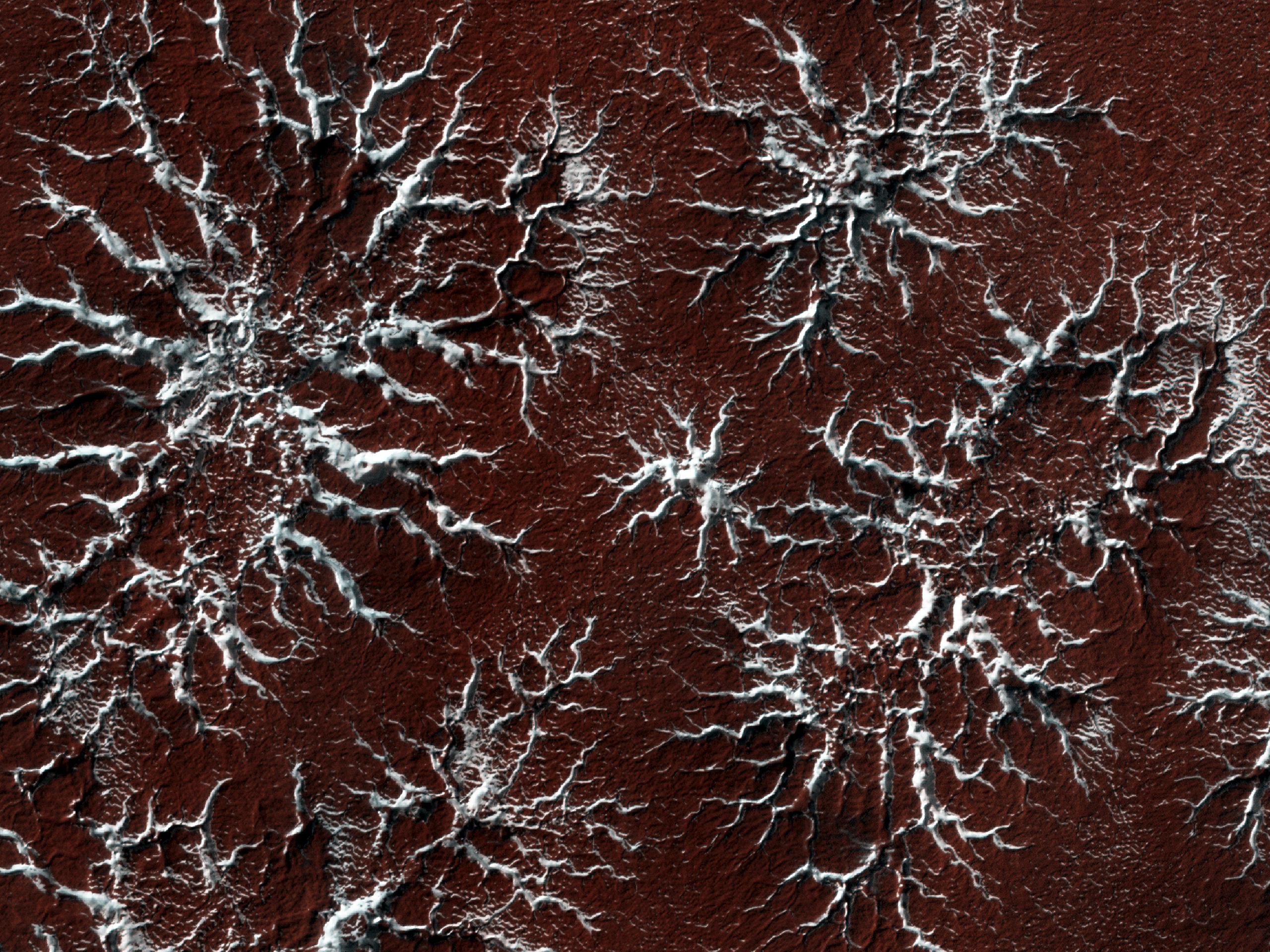
The image above was dry ice on mars as you can see it is not clean even if you are using a very large plate for it to fall onto....
For mars water is in the air and it falls just as the co2 does but at different temperatures one then the other in think layers with each other.
The poles have a mixture of these with the dust falling with in these layers. Its not going to be pure....
That is energy in use for a pure atmospheric removal, filtering of dust allowing it to deposit onto a plate during the night that is cooled to create a more pure co2 dry ice but even that will have some water and dust even with filtering.
The term ice has nothing to do with dry and is water here on earth with some dust particles or minerals depending on the source of the water that will make it as its cooled to below freezing...
Now dirt and accumulating dust would be bad within a reactor core for the rods to make contact to, as that would block the cooling and heating effects of the liquid co2 that is used to make the co2 steam plant section of the design work. We feed liquid into the bottom and as it heat it rises to form co2 steam that is vented into the turbine loop to create power.
Offline
Like button can go here
#20 2020-09-12 17:06:37
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,514
Re: Liquid Co2 reactor cooling
tahanson43206,
The Martian atmosphere is heavily loaded with a fine abrasive dust that coats everything it touches. Static electricity from the incredibly dry environment ensures that it adheres to everything it touches. You're not going to get away with running an abrasive volcanic dust through a turbine because it will abrade the leading edges of the turbine blades and clog bearing housings in short order. The CO2 has to be separated from the dust in order to use it. One way to do that is with centrifugal force, but there are other ways, as you noted. If you could collect CO2 without sucking in Martian atmosphere through a turbine, then you could heat the collected CO2 to turn it into a gas and the dust would settle in the bottom of the collection tank where it could be removed, as you suggested doing. There are probably time limitations on the settling out of the dust loading, though. It may take several hours, or maybe not, but you'd have to test it with a realistic simulant to know for sure. To limit turbine damage from dust ingestion, I think we need a closed loop system that uses thermal power from the Sun with pure CO2 collected from the environment, through whatever method.
Offline
Like button can go here
#21 2020-09-12 17:18:00
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,100
Re: Liquid Co2 reactor cooling
For kbd512 (#48) and SpaceNut (several posts) ....
Since Calliban proposed the reactor design, I am hoping he will return to defend his design from the concerns listed.
This topic is about CO2 sublimination. It is ** not ** about water, in any shape or form.
Water is covered extensively in other topics.
Edit#1: It would help a ** lot ** if there were actual data from probes to answer the questions raised about the quality/purity of the dry ice covering the landscape on Mars.
I think it is perfectly reasonable to suppose that dry ice can and does precipitate on the landscape in layers that are free of contaminants. In order for the material to precipitate (I am assuming) the air must be still. If dry ice accumulates in layers meters thick, it should be possible to harvest the material without scraping up the regolith.
(th)
Last edited by tahanson43206 (2020-09-12 17:23:11)
Offline
Like button can go here
#22 2020-09-12 17:33:57
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Liquid Co2 reactor cooling
The concept design, as it stands, is for a once-through reactor. Liquid CO2 is produced by loading the impure mixture of ice, dry ice and dust into external retorts. These are then heated to -50C. The CO2 liquefies, whilst the ice remains frozen. The liquid CO2 may need to be filtered to remove any suspended dust contaminants, before injection into the reactor. However, CO2 is non-polar, so liquid CO2 should not carry any dissolved salts which could act as neutronic poisons if they get into the core. Low levels of these poisons could be tolerated. But the liquid CO2 must be relatively pure, as the reactor runs on natural uranium and neutron economy is already quite tight. To remove any residual ionic compounds, the system could be fitted with a carbon filter.
CO2 would flow up the aluminium fuel channels, being heated to a temperature of 30C (just beneath critical temperature). The liquid flowing out of the top of the fuel channels would enter a vapour separating tank. This would be filled with boiling liquid CO2. The static pressure in the fuel channels would prevent the CO2 from boiling until it enters the tank. A temperature gradient would exist within the liquid within the tank. Liquid at the top of the tank would be boiling at 30C. Liquid at the bottom of the tank would be several degrees lower and would remain liquid. Injection pumps would suck liquid CO2 out of the bottom of the tank for injection into the fuel channels. CO2 gas drawn from the tank would pass through the turbines and be vented up the stack. Residual heat from the CO2 gas will be drawn on to keep the retorts at -50C, prior to venting up the stack. This would likely make use of an intermediate heat exchanger.
Liquid CO2 will be injected into the vapour separation tank from the retorts by a centrifugal pump, at a pressure of about 80 bar. It would keep the level within the tank constant, as vapour is drawn off. It would also maintain thermal gradient within the tank.
An alternative design would be a graphite moderated, pressurised water reactor, with water flowing through the aluminium cooling tubes. This would be rather like the RBMK reactor, except the water would remain liquid within the pressure tubes. There is no danger of a Chernobyl type accident, because water is not boiling in the channels. As the amount of water used in cooling is relatively small, the reactor would still work with natural uranium. The water would flow into a heat exchanger within a boiler, which would boil the liquid CO2 injected from the retort. The advantages of this design are that: (1) It avoids any problem of contaminants in the liquid CO2 stream; (2) The water can be kept liquid at much higher temperature than CO2, hence the power cycle will be much more efficient; (3) At 100C, the vapour pressure of water is only 1bar, so the primary circuit pressure is much lower and aluminium fuel channel tubes can be much thinner. In fact, if the graphite stack is pressurised to ~1bar, there need be no net pressure in the fuel channels.
The downside is that a heat exchanger is needed. That adds cost to the design. However, given the other advantages to the design, it might be better to keep the CO2 in a secondary circuit, with a water cooled primary circuit.
Last edited by Calliban (2020-09-12 18:02:05)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#23 2020-09-12 18:42:29
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
I separated the liquid co2 reactor content from the co2 sublimation dry ice topic...
Offline
Like button can go here
#24 2020-09-13 07:28:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
The CO2 is located at the polar regions where there is no natural heat source at all for half of the Martian year. For the rest of the year, there is weak sunlight - with intensity of 43% of what is found at Earth polar regions. That is why it is frozen.
Whilst dry ice is a convenient working fluid for an engine, there still need to be an input heat source to generate power. It is not a free energy source, it is a phase change material that will only generate power from whatever energy you pump into it. This cannot realistically come from the sun at the Martian poles. Geothermal energy is a weak possibility, though the geothermal gradient in the Martian crust is shallower than Earth's, meaning that we would have to drill much deeper.
That means more cost and complexity than already face here on Earth. So it follows that any sublimation heat engine on Mars will be powered by nuclear energy, given that there is no other practical heat source. So its really a question of how to do that best and what type of reactor we could practically build with the resources that we can afford to access.
Which is why the poles dry ice is so contaminated.
Offline
Like button can go here
#25 2020-09-13 07:29:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,577
Re: Liquid Co2 reactor cooling
For Calliban re reply to Louis ...
It is good to see Louis back on the scene, in full support of solar power and in full opposition to nuclear fission.
It is ** equally ** reassuring to see your robust defense of nuclear fission at the poles of Mars. I like the vision I can build in my mind's eye, of robust communities at the poles supplying all manner of goods and services to customers in lower latitudes.
A concern I do have is supply of fissionable materials. A quick review of Google snippets on the subject indicates that while there ** are ** some signs of Uranium and Thorium on Mars (evidence from satellites, primarily) I would imagine it would take some effort to collect enough from the regolith to deliver useful power in the quantities needed for a productive community.
It is in the collection of fissionable materials that I would think solar power would prove useful. Production can take place as power is available, at unattended facilities.
Here is a solution that allows both solar power and fission to find useful employment in a hostile environment.
(th)
The thorium for a nuclear power heating was added to post #3 for local source rather than importing it from earth.
Offline
Like button can go here