New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2025-12-21 16:34:01
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,559
Re-purposing Moxie for Co + O2 Fuel
For SpaceNut re Moxie....
The Moxie experiment runs with energy from a radioactive generator.
Please consider asking your AI friend to figure out how many Moxie units would be needed to provide the oxygen for the crew for that yearlong stay.
Please note that your report stated that carbon monoxide is produced by Moxie. That ** is ** a fuel! There is NO need for anything more fancy that that.
How many Moxie units would it take to produce the fuel and oxidizer for launch that is equivalent to the 50,000 ton figure your report mentioned.
(th)
Online
Like button can go here
#2 2025-12-21 18:03:18
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,967
Re: Re-purposing Moxie for Co + O2 Fuel
Moxie used just 300 Watts to do this experiment from the radioactive generator.. The system while it may scale does not mean that its going to process even when fully tested here on Earth.
The Co is only part of a fuel as it needs an oxidizer to make it useful.
The equation for converting CO₂ to O₂ and CO involves the decomposition of carbon dioxide, often requiring energy, and the balanced form for producing both is complex, but for simple decomposition into solid carbon and oxygen, it's \(2CO_{2}(g)\rightarrow 2C(s)+2O_{2}(g)\) (or simplified \(CO_{2}\rightarrow C+O_{2}\)), while the reverse, forming CO₂, is \(2CO+O_{2}\rightarrow 2CO_{2}\). For a reaction that yields both CO and O₂, it's a partial combustion of carbon, like \(2C+O_{2}\rightarrow 2CO\), or a mix, but you can have \(C+O_{2}\rightarrow CO+CO_{2}\) (which balances to \(2C+2O_{2}\rightarrow 2CO+CO_{2}\) or \(C+O_{2}\rightarrow CO+\frac{1}{2}O_{2}\) if you want CO + O₂). Key Reactions Complete Combustion (CO₂ Formation): \(2CO+O_{2}\rightarrow 2CO_{2}\) (Carbon monoxide + Oxygen \(\rightarrow \) Carbon dioxide).Partial Combustion (CO Formation): \(2C+O_{2}\rightarrow 2CO\) (Carbon + Oxygen \(\rightarrow \) Carbon Monoxide).Decomposition (CO₂ to C + O₂): \(2CO_{2}\rightarrow 2C+2O_{2}\) or \(CO_{2}\rightarrow C+O_{2}\) (Carbon dioxide \(\rightarrow \) Carbon + Oxygen).Mixed Products (From Carbon): \(2C+2O_{2}\rightarrow 2CO+CO_{2}\) (Carbon + Oxygen \(\rightarrow \) Carbon Monoxide + Carbon Dioxide). Balancing Example (CO₂ \(\rightarrow \) C + O₂) Unbalanced: \(CO_{2}\rightarrow C+O_{2}\) (1 C, 2 O on left; 1 C, 2 O on right - Wait, the O is split!).Balance Oxygen: You need 2 O on the right (from \(O_{2}\)) and 2 O on the left (from \(CO_{2}\)).Final Balanced (Simplest): \(CO_{2}\rightarrow C+O_{2}\) (This looks balanced but often needs coefficients for real-world application, like \(2CO_{2}\rightarrow 2C+2O_{2}\) to ensure whole numbers if you are breaking down multiple molecules). The specific equation depends on what you're starting with (CO or C) and what you want as products.
The balanced chemical equation for the decomposition of carbon dioxide (\(\text{CO}_{2}\)) into carbon monoxide (\(\text{CO}\)) and oxygen (\(\text{O}_{2}\)) is:
2CO[sub]2[/sub] → 2CO + O[sub]2[/sub]This equation can also be represented in BBCode format using HTML-like tags for subscript:
2CO[sub]2[/sub] -> 2CO + O[sub]2[/sub]
Offline
Like button can go here
#3 2025-12-21 21:09:01
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,559
Re: Re-purposing Moxie for Co + O2 Fuel
For SpaceNut .... the oxidizer for CO is produced by Moxie!
Moxie produces fuel and oxidizer by separating CO2 into O2 and CO.
There is NO need for an additional oxidizer. This means that a single MOXIE device can produce fuel and oxidizer for any purpose on Mars.
There is NO need to create a higher order fuel molecule using hydrogen, unless there is something that calls for higher stored energy.
ISP of 250 should be acceptable for MOST operations on Mars, including reaching orbit.
MOXIE does NOT need an external power supply. I has it's own power supply.
MOXIE does NOT need to be tested on Earth. It is (was) tested just fine on Mars.
Your AI friend should be able to confirm all those points, if you give it the right query.
My point was (and still is) that MOXIE does the job, so why not just reproduce it as many times as necessary.
If you need a ton of oxygen to care for your crew of 4 for one year, how many MOXIE devices do you need?
Why invent something else? I just don't see the point.
(th)
Online
Like button can go here
#4 2025-12-22 15:23:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,967
Re: Re-purposing Moxie for Co + O2 Fuel
I think that I miss read you post as it appears that the life support for humans is not what you are asking about.
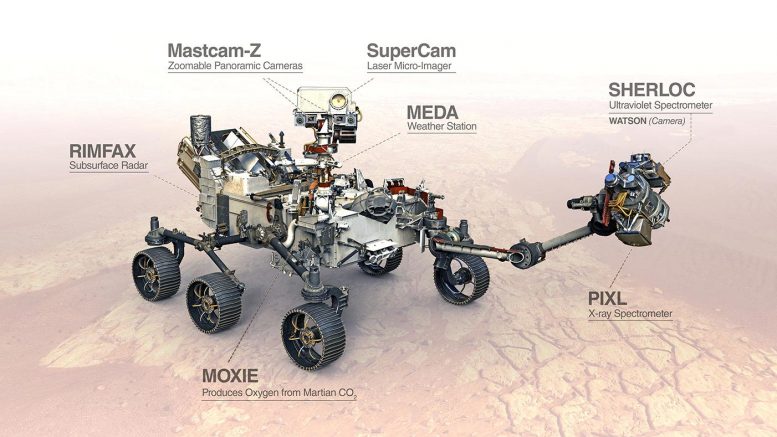

Gold box being positioned over the rover.
The unit rides currently on Perseverance which supplies power from its RTG as electrical for the unit to work at 300 watts. As the unit does not have its own power system or supply.
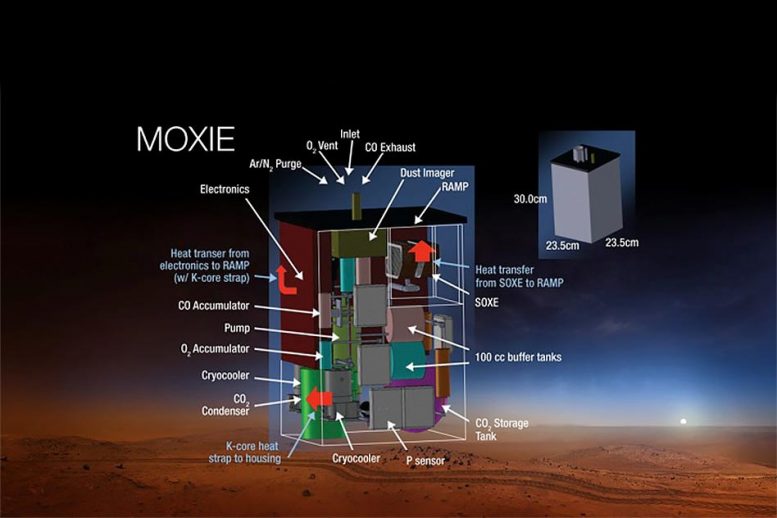
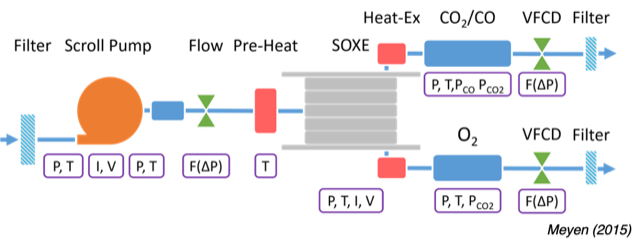
The main parts are a compressor to draw pair in, the co2 electrolysis unit ( Solid OXide Electrolyzer (SOXE),). and lots of sensors and filters.
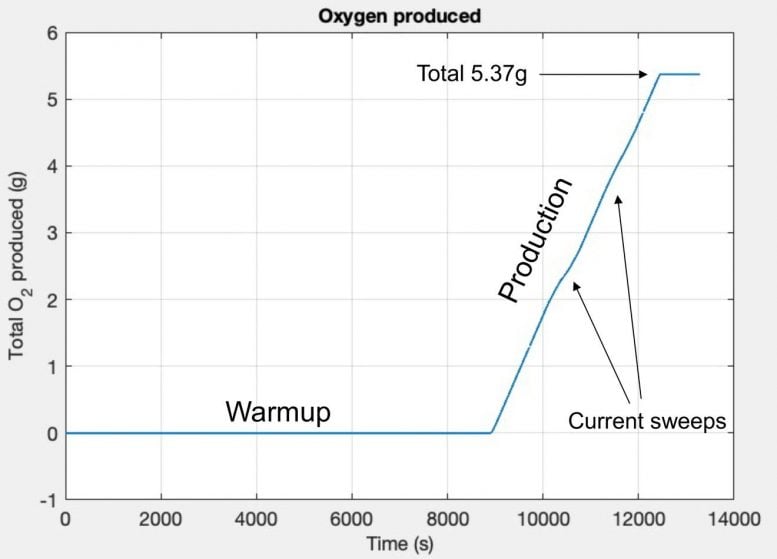
NASA's MOXIE on Mars produces about 6-10 grams of oxygen per hour (like a small tree) after a warmup, with runs lasting around 3.5 hours (2.5 hrs warm-up, 1 hr production). The initial target was 6g/hr, but it exceeded goals, reaching up to 12g/hr at peak performance, proving scalable technology for future human missions by converting Martian CO2 into oxygen.
MOXIE's Performance & Process:
Standard Output: Around 6-10 grams of oxygen per hour (g/hr).
Peak Output: Achieved up to 12 g/hr, doubling initial targets.
Typical Run: About 3.5 hours total (2.5 hrs warm-up, 1 hr oxygen production).
Total Produced (by late 2021): Over 100 minutes of breathable oxygen for an astronaut, or 50 grams.
Technology: Uses Solid Oxide Electrolyzer (SOXE) at 800°C, splitting CO2 into oxygen and carbon monoxide.
Significance:
Demonstrates In-Situ Resource Utilization (ISRU) for Mars colonization.
Knowledge gained will inform future, scaled-up MOXIE systems needed for crewed missions, potentially producing kilograms of oxygen
Mars Oxygen ISRU Experiment (MOXIE)—Preparing for human Mars exploration
It cycled 16 times during night and day with the pressure measure with temperature with timed duration for oxygen creation.
It does not run continuous but must cycle to make it not become damaged.
It has no storage tanks for the exiting gasses which will check values, pumps to pressure rise the amounts stored.
KBD512 pg 14 post gave a scaled up post in the Internal combustion engines for Mars which is theoretical as no vehicle has had it done to the engine. Tank size is dependent on how long you wish to use the fuel but also how much it can carry on its frame.
We also do not have a production rocket engines that I could find. Plus the rockets that we are using to get to its surface is a CH4+O2 engine.
Carbon Monoxide and Oxygen Combustion Experiments: A Demonstration of Mars In Situ Propellants
Offline
Like button can go here
#5 2025-12-22 15:29:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,967
Re: Re-purposing Moxie for Co + O2 Fuel
The largest solid oxide electrolyzer (SOE) developed for Mars is the mission-scale SOXE stack by OxEon Energy, a 33x scaled-up version of the toaster-sized MOXIE device on NASA's Perseverance rover, designed to produce propellant for human return missions, though the MOXIE unit itself was the first SOE to operate on Mars, generating oxygen from CO2. On Earth, Bloom Energy's 4 MW Bloom Electrolyzer is the world's largest SOE system, stemming from that original NASA Mars technology.
For Mars Missions (Technology for Future Human Exploration):
MOXIE (Mars Oxygen ISRU Experiment): This small, toaster-sized SOE device on the Perseverance rover successfully demonstrated solid oxide electrolysis on Mars, producing oxygen from the thin CO2 atmosphere.
OxEon Mission-Scale SOXE: OxEon scaled up its MOXIE technology significantly (33x) for potential Mars crewed missions, aiming to produce large quantities of oxygen for propellant (Mars Ascent Vehicle) and life support, according to this TTU DSpace Repository document and OxEon Energy's website.
For Earth (Current Largest SOE):
4 MW Bloom Electrolyzer: Bloom Energy built the world's largest solid oxide system, operating at NASA's Ames Research Center, with roots in the original Mars technology to produce clean hydrogen for terrestrial decarbonization.
In summary, MOXIE was the first SOE on Mars, OxEon scaled it up for future large-scale Mars needs, and Bloom Energy created the largest SOE on Earth based on that heritage
The cell can also be used to make power from the input at about 60% efficiency.
Offline
Like button can go here
#6 2025-12-22 16:02:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,967
Re: Re-purposing Moxie for Co + O2 Fuel
For SpaceNut re new posts in MOXIE topic ...
Thanks for the new posts you added, with what sure looks like ** very ** encouraging news of larger scale operations on Earth.
My point was (and remains) that the original small MOXIE unit could be replicated in large numbers and deployed to Mars with an RTG able to provide the 300 watts it needs. It does NOT need to be mounted in a rover. That was convenient for the research mission.
Your posts show that researchers/engineers are working on larger scale versions.
That is all good, but the fact remains, the ONLY such system tested on Mars is the 300 watt version.
So we should be able to figure out how many 300 watt units are needed to supply oxygen for humans or fuel and oxidizer for machinery.
***
In one of your posts you seemed (as I read it) to think that carbon monoxide had never been tested on Earth for internal combustion engines. I am 95% confident that kbd512 researched that long ago and found that such engines had most definitely been tested on Earth.An internal combustion engine that runs on CO and O2 will produce less power than would an engine that has hydrogen in the fuel, but I question why that makes a difference. To make hydrocarbon fuels will consume energy that you might be able to get back if you have an engine designed for it, but why bother? CO and O2 make a perfectly acceptable energy storage system and the whole process is so much simpler, I just don't see why anyone would go to all the trouble of fooling around with hydrogen.
(th)
Offline
Like button can go here
#7 2025-12-22 16:06:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,967
Re: Re-purposing Moxie for Co + O2 Fuel
If the question are put into the topics then one can make better sense of what is required.
Astronauts can approach an operating Radioisotope Thermoelectric Generator (RTG) very closely, even working in its immediate proximity, but the exact safe distance is determined by mission design, the specific RTG's shielding, and the total duration of exposure, as part of strict radiation safety protocols.
Safety limits are based on the principle of keeping exposure As Low As Reasonably Achievable (ALARA) and not exceeding established dose limits.
Key Safety Principles
Distance is a Primary Shield: Radiation exposure decreases significantly with distance. Doubling the distance from a source can reduce the exposure rate by a factor of four (inverse square law).
Mission-Specific Limits: NASA sets specific limits for radiation exposure from nuclear technologies, which are typically capped at an effective dose of 20 millisieverts (mSv) per mission year (prorated for mission duration). This limit is designed not to add more than 10% to the unavoidable background space radiation dose of a mission.
Shielding: RTGs used in space missions are designed with shielding to minimize external radiation. For planetary surface operations (like on Mars or the Moon), the mission plan factors in habitat shielding, terrain as a potential shield, the distance from the source, and the duration of extravehicular activities (EVAs).
Time: The duration of exposure is strictly managed to stay within short-term and career dose limits.
Practical Application
In practice, astronauts on Apollo missions worked near the deployed RTGs of the Apollo Lunar Surface Experiments Package (ALSEP). They would align the ALSEP so that the RTG's cable exit pointed toward the central station, and while they avoided unnecessary proximity, no specific maximum distance constraint was imposed in the same way as avoiding a rocket launch, due to the contained nature and shielding of the RTG's radioactive material.
For future missions, safety zones and operational procedures are established through rigorous analysis to ensure that cumulative and acute radiation doses remain within safe, regulated limits for all crew members
Mars has quite a bit of radiation already for a crew to deal with so additional shielding would be needed.
The other is mass shipped to mars is not sustainable.
Offline
Like button can go here