New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#501 2025-01-11 14:49:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,061
Re: Venus
From today's NSS Zoom presentation:
Per Dr. Greg Stanley:
Rocket Lab is planning a launch to Venus in January of 2025.
The slide containing the details flipped past before I could get logged in to copy the details.
However, the details should be available for lookup on the Internet.
The probe is (apparently) designed to search for signs of life on Venus.
(th)
Offline
Like button can go here
#502 2025-01-11 16:37:21
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I would not have a hard time bypassing Venus, if life is discovered.
Ending Pending ![]()
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#503 2025-01-12 18:21:48
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,483
Re: Venus
The How
Magnesium metal powder imported from Earth will combust in a pure CO2 atmosphere, leaving behind Carbon and Magnesium oxide. Magnesium has such a strong affinity for Oxygen that it will readily strip Oxygen from CO2, leaving pure Carbon powder behind. This is a highly exothermic reaction, which produces temperatures of 2,200C to 2,600C. This could supply some of the heat required to reprocess a previously reacted batch of MgO, back into Mg metal.
2Mg + CO2 → 2MgO + C
CO2 = 44.009 g/mol; 22.7226mol/kg
Mg = 24.305g/mol; 41.1438mol/kg
12.011(C) / 44.009 (CO2) = 0.27292 <- Percentage of CO2 mass consisting of Carbon
We need 3.665kg of CO2 to produce 1kg of pure Carbon fuel powder.
We need 3.261kg of pure Magnesium metal powder to completely combust 3.665kg of CO2.
If we want to make batches of Carbon powder fuel corresponding to the energy-equivalent of 1,200 gallons of diesel fuel, when combusted in a 70% thermally efficient Allam-Fetvedt cycle driving a sCO2 power turbine, then we need to import at least 5,429.565kg of Magnesium metal and 6,102.225kg of pure CO2 collected from the Venusian atmosphere.
I have found varying fuel tank capacity figures from Caterpillar. The 2007 document I have from Caterpillar on the 797F mining truck lists a fuel tank capacity of 1,000 gallons of diesel fuel. Wikipedia lists a fuel tank capacity of up to 2,000 gallons, but I haven't found any Caterpillar spec sheets listing a 2,000 gallon fuel tank capacity. The Caterpillar 6060 hydraulic excavator has a fuel tank capacity of 3,136 gallons of diesel fuel. The Cat 6060 is the largest excavator that Cat makes, just as the 797F is the largest mining haul truck that Cat makes. There are a literal handful of mining haul trucks capable of hauling more ore, or hydraulic excavators with larger bucket capacities, but all of them are much larger / heavier, and very few of them have been made, relative to the 797 series.
The smallest credible industrial scale mining operation would include two excavators, six ore hauling trucks, a rock comminution plant, an ore flotation plant, and a smelter to make metal. The truck and shovel fleet requires 18,692kg of Carbon powder fuel per day to run a 24/7 operation, so 60,956kg of Magnesium powder must be combusted with 68,508kg of collected CO2. We probably need at least twice as much Magnesium powder so that one batch of fuel can be made and then the following day the MgO can be coverted back to Magnesium metal, ground into a fine powder, and then fed back into the reactor used to make Carbon powder.
Going the other way with the MgO, back to pure Magnesium metal, requires temperatures over 2,852C:
2MgO → 2Mg + O2
We need sufficient electricity and a vacuum arc furnace to achieve 3,000C, and perhaps some waste heat from the first reaction used to produce the Carbon powder fuel. MgO starts to thermally dissociate at temperatures above 2,852C, which corresponds to MgO's melting point. We're melting about 2,540kg of MgO per hour.
MgO's specific heat capacity is 877J/kg·K, so (3273.15K - 755.15K) * 877J = 2,208,286J/kg, or 5,609,046,440J / 1,558,069Wh of energy to melt 2,540kg of MgO per hour. With an 85% thermally efficient induction heater / furnace, 1,833,022Wh. Let's call it a 2MWe requirement to reprocess the MgO and collect the oxidizer required to make the fuel. Our thermal updraft tower, must therefore be a 2MW device. Ideally, we would want two of those as well. This is an industrial mining application, after all, and we can't tolerate much down time. Our equipment must run 24/7 to make money for the company.
A functional space-rated small modular nuclear reactor would make all of this a lot easier to accomplish. There is no denying that simple fact. Unfortunately, nobody seems to be working towards commercialization of the tech in a serious and coherent way. There are pockets of marvelous ideas, as it pertains to nuclear power, for both terrestrial and space applications, with insufficient funding and nothing approaching a "program of record", in government-speak. That is why I'm searching for viable alternatives that really could work because they pass all affordability and materials availability "basic math sanity checks", and fall solidly within the realm of "engineered technology solutions". The sCO2 gas turbine is an application of existing gas turbine engine tech that fundamentally works quite well, uses less materials to make and power it than all competing solutions except nuclear power, and it provides a power density second only to chemical rocket engines.
What do I mean by "program of record"?
I mean there is no apparent consensus on going after a new form of nuclear energy that is more "user friendly" for the plant operators and more broadly applicable to powering applications beyond entire cities or large ships. When it comes to city-scale commercial power reactors, there is near-universal acceptance that pressurized water reactors are the technology of choice. Even if they're not the most user-friendly of reactor designs, they represent the lion's share of civil and military power and propulsion reactors. Everyone who operates nuclear reactors operates at least one of these power plants, if not several, because they're so well-understood. The institutional knowledge of how to operate an over-glorified steam kettle is pervasive. We know how they fail, and for the most part, if we do our part, they work as advertised.
The What
What metals do we want to "go after" on Venus?
Lead, Antimony, Bismuth, Copper, Manganese, Titanium, and Magnesium are all present on the surface. The MgO on Venus is high-value to the mining operation itself, because it can be used to produce more Carbon fuel for the vehicle fleet, in order to expand operations.
Lead is require to make bullets and batteries. If those Martians ever invade, we'll be ready for them. We're clearly never going to make enough Lithium batteries. If we had access to a lot more Lead and Copper, though, then my guess is that we'd settle for Lead-acid batteries here on Earth. They're easier to use and recycle than Lithium, and seem to catch fire far less frequently.
Lead-crystal (Lead-glass / Lead-oxide) batteries are currently re-vamping Lead-acid battery technology, with Silicon dioxide (2,500 cycles) and Carbon foam (3,500 cycles), at 50% depth-of-discharge. They contain very little Sulfuric acid. This is well beyond what traditional Lead-acid batteries are capable of. Most importantly, they are almost completely recyclable. The electrolyte is almost solid, so it's a near-solid-state battery. It's also a mass-produced commercially available product.
A Tesla PowerWall 3 costs $13,500 and stores 13,500Wh of energy, and it only weighs 287lbs, so 47.04Wh/lb, and 7,888.8in^3 in total volume.
You can purchase an 18,400Wh Lead-crystal battery for $3,698. I'm sure delivery to your home will cost $500-$1,000, because it weighs 898.4lbs, so only 20.48Wh/lb, and 6,501.6in^3 in total volume. However, it's a clear undeniable winner on cost and total system volume, even if a separate charge controller is included. There are just 8 Lead-crystal battery cells in the pack, vs a bare minimum of 925 individual 21700 cells for the PowerWall 3, at 14.6Wh per cell. The relative complexity of both battery technologies is crystal clear. Tesla PowerWalls look like Mac computers inside. All that tech is obviously doing something. Tesla didn't put it there for no particular reason. Unfortunately, you as the owner-operator probably have no clue what it's doing. That means you're never going to repair it if something fails. The Lead-crystal battery is a small black box that weighs about 51kg per cell. You need two strong men to pick one up and move it with ease, but it has a pair of terminals on top and that's about it. If you have a normal car battery tester, then you can figure out if it's safe to use. You're going to need a lot more hardware and software to evaluate a PowerWall's usability. Some of us like simple things that just work without a lot of fussing and messing around. When I buy a battery, I want to buy a good battery at a good price, not a world-class computer with hundreds or thousands of tiny batteries connected to it. PowerWalls are great products, and I presently own and use two of them, but after looking inside one of them, I can recognize when I'm out of my depth. If I had 8 Lead-acid batteries, any installation, repair, or maintenance work would be understandable. Most of the calcs, such as the gauge of the wiring required, I can do in my head, and the rest I can do with a pocket calculator and a table of input values. Trying to do the same thing with a Lithium-ion battery packs?... Get real.
I think we need more Lead and Copper if we want to use more electricity for cars and less combustion. We don't have enough of those materials here on Earth, so it means we have to get it from somewhere else. I'll bet almost anything that Venus is absolutely loaded with heavy metals like Lead, Antimony, Bismuth, Tin, and Copper. It probably has Iron and Aluminum coming out the wazoo, but we're not short of either and probably never will be. If someone told me, "slow your roll on ripping up the Earth to get more metal", then I would go to Venus first, then Mars, and finally out into the main belt if I couldn't get enough metal from Venus and Mars.

Copper's uses are fairly obvious. There is no electric or electronic anything without Copper.
Manganese is required to make economical Mangalloy steels for transport of cryogenic liquids in bulk quantities. Titanium would be a good alternative to steel if we could source enough of it.
Antimony and Bismuth is required by pharmaceuticals, cosmetics, rubber production, nuclear medicine, fuses, and semiconductors / resistors. Bismuth's most important use for our mining application is making Caterpillar's iconic yellow paint. You can't have a proper Cat-driven mining operation without the yellow paint.
Antimony is used to make Lead harder, particularly for bullets, Lead-acid batteries, increasing corrosion and wear resistance in metal alloys, and other applications broadly similar to Bismuth. Antimony is a catalyst used to make PET plastics, and we have an insatiable appetite for those.
The Why
What all that metal can do for humanity is a good enough reason for going to Venus and spending real money to transform it into a mining planet. We will then have access to more metal than has ever been mined on Earth. Modern human civilization is built on metal and concrete. If we tear up the entire planet to get more metal, then Venus is no more or less habitable than it was before. Venus and Mars don't have a habitability issue. Anyone living there is using energy and materials technology to survive. If we tear up every bit of the crust of Venus to get more metal, nobody outside of academia will care, because absolutely nothing is presently living there. Humanity needs more metal to give everyone on Earth, even the people who choose to live on other planets, a "modern lifestyle" where food, materials, and energy are all abundant and available to everyone.
The Africans aren't tearing up the ground in Africa, the way we did here in America 100 years ago, because there's plenty to be had. They know that energy, mining, and manufacturing is how absolutely everyone escapes from the crushing poverty that persisted for most of human history. It's a simple plan that happens to work. There would not have been any famines in ancient Egypt if they had water desalination, combine-harvesters, fertilizers, and waste water treatment plants. I don't think the humans of antiquity were any less intelligent or less capable than we are today. They lacked knowledge, but all knowledge came about by people who were able to dedicate their lives to its pursuit. Apart from technological innovation, to make all of that "stuff" we have today, you needed to have specialization of labor, in addition to energy and materials. To unlock labor specialization, you require sufficient energy and materials so nobody starves in the street, except by choice.
If all the raw materials can be imported from places where they are incredibly abundant, then available farming land becomes the only limiting factor. If you can grow food in space or in the oceans, then energy becomes our only limiting factor.
Prosperity is not a given. You can't simply throw money at any particular problem and expect it will solve itself. Prosperity must be actively pursued at all times, endlessly, using a coherent strategy formalized into a "program of record". Right now, our society and way of life depends on energy, ever-increasing quantities of resources we can transform into new materials, and food / water / air. So long as we're still human in nature, it's highly likely that this always will be what we require.
Our first coherent strategy objective for this program of record is to produce the off-world mining and materials transport technology required to expand our readily accessible natural resource pool so that what we can tap into is not limited to Earth itself. If we have access to all the metal resources between Mercury and the Main Belt, then our metals inventory is effectively unlimited if compared with what we are presently working with. Metal is pretty near to a hard requirement for construction, especially in space. More advanced materials can be used in certain cases, but Iron / concrete / glass represents the technology floor, below which expansion of human civilization through colonization and industrialization is not practical.
After technology development is sufficient, we start the implementation phase of this program, which goes after the highest-demand / lowest-supply resources closest to Earth. We need more Copper, Tin, Lead, and Antimony if nothing else. This is less about price than it is about total available supply. We're not building new mines because we've pretty well cleaned out the accessible resources. For us to obtain a lot more Copper from Earth itself, we're talking about leveling the Andes Mountains. That's an absurdity, even if we have the energy / labor / capital to do it. We need to conduct comrpehensive surveys of Mercury, Venus, Mars, the moon, and previously idenfitied Main Belt objects likely to become valuable resources in the future. To wit, "1986 DA" (PGMs / Nickel / Gold / Copper), "16 Psyche" (PGMs), and "6 Hebe" (Copper; estimated at up to 1.39 trillion tons- enough to allocate 139 tons to every man / woman / child with a population of 10 billion people, so probably enough for 100% electrification) have the materials we're after in incredible abundance. To avoid any new wars over these resources, that's how we're going to allocate the material. Every nation-state gets a population-proportionate allocation of material. We're going to simply give it to everyone, and they're going to pay only what it costs to go get it and bring it back to Earth / Mars / whichever planet we colonize next.
Finally, we begin to move all mining activities off-Earth whenever depletion starts to become a real problem. This is already the case for Gold, Silver, Copper, Tin, and Lead. Prices remain relatively low, but it's not as if we can easily "get more" if we need more. That is the specific economic problem that this program of record aims to solve. Whenever we need more, we contract with companies to go and get more, and they're responsible for working out the details of how to best accomplish that.
Problems Caused by Metal Availability and Cost
In California, the plastic piping in homes directly contributed to wildfires spreading after the fires melted / burned through plastic plumbing piping, rupturing the water mains, and causing water pressure to be lost when it was required for firefighting. If Nickel-Iron piping was cheaper than plastic because we were hauling huge quantities of it back from the other planets and Main Belt, then there wouldn't be much of a market for making weak PVC / Lead / Copper plumbing piping that easily melts during a fire. Nickel-Iron piping won't melt during a fire, it's corrosion resistant, and a Silicon-based corrosion control surface coating could make it last much longer than a human lifetime, potentially for centuries.
When comparing service life, iron piping (typically cast iron) generally lasts the longest, with a lifespan of 80-100 years, followed by copper pipes which can last 50-70 years, while plastic pipes (like PVC) have a shorter lifespan, ranging from 25-50 years depending on quality and installation conditions.
Offline
Like button can go here
#504 2025-01-12 20:17:10
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
Interesting materials. Frankly so much that my ADD kicks in, so I hope I won't offend you.
In addition to what you have proposed, I suggest pyrolysis:
https://en.wikipedia.org/wiki/Pyrolysis
If gobs of biomass could be produced in the clouds, then Algae and Cyanobacteria could be subjected to some of the lower temperature pyrolysis processes with the natural heat of Venus.
From this post, #494, I have this simple suggestion for growing the biomass: https://newmars.com/forums/viewtopic.ph … 08#p229008
Quote:
In the clouds I have this proposal:
In the pyrolysis, article I included above, there appear to be several pathways to various types of fuel.
I might take your idea about LOX, and suggest putting the biomass into a drum, and possibly a low quality fuel would be made available simply by heating the contents of the drum. Possibly a turbine or motor could run off of these fuels with could be syngas, oils, and Carbon. I suppose that in reality you might want to make more refined products.
In addition to LOX as a coolant, you might also have Liquid Nitrogen and Liquid CO2, and those also could be used as coolants on an aircraft, even turning motors as the situation heats up at a low depth in the atmosphere of Venus.
Your surface mining equipment might be able to receive some of these products.
Ending Pending ![]()
Last edited by Void (2025-01-12 20:27:35)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#505 2025-01-13 13:58:04
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,483
Re: Venus
Void,
Are you suggesting we make a liquid fuel like diesel?
Petroleum products made from algae could become useful energy stores for airship engines, operating in the clouds above Venus. However, for use at the surface, the issue becomes the flashpoint of the fuel. 482C is WAY above the flashpoint of tar-like substances. Tar has a flashpoint ranging between 150C and 300C, so we'd need a pressure vessel to store tar at 482C, assuming that's possible without thermal breakdown, or some way to insulate and cool fuel tanks. Pure Carbon has an auto-ignition point of about 700C in "air" and 660C in pure O2.
It is obviously easier to work with gaseous or liquid fuels, even a tar-like substance for that matter, when compared to Carbon / coal dust, but storing a gas or liquid fuel at the ambient surface temperatures would prove quite challenging, mostly because I think any kind of feed lines and valves would get gummed up from polymerization or coked up.
Feeding a fine particulate fuel into the combustor, in the form of a fluidized powder that's been pre-mixed with CO2 for both pressurizatio and to keep temperatures under control, is probably an easier proposition, even though that still poses coking challenges when the powder hits that very hot combustor can.
Is there going to be some kind of heat sinking strategy to keep the fuel at usable temperatures, perhaps mixing with "cold" CO2 at 250C, or is this fuel something to power airborne vehicles?
Tar / bunker fuel is much easier to combust than a solid like Carbon and won't have any molten chunks of Carbon impinging on the tubes of sCO2 being heated, but if this algae-supplied bunker fuel is to be consumed at the surface, then it's use / lose without active cooling. This may not be an insurmountable problem, though, because these mining machines will eat fuel like candy. If you can devise some kind of continuous production process, that is preferable to a batch process with coal production, even though it's more complex. Real world oil refineries back here on Earth work on continuous production principles, because the output can be continuously varied to meet immediate demand.
How would you keep the product just below its flashpoint, and how do you optimize your production process to produce as much tar as possible and as little of everything else?
You're not going to be able to "keep" LOX at the surface in any practical way, which is why I specified highly pressurized "hot" O2 storage tanks for the mining vehicles, which is part of what makes them so heavy. There's going to be a minor temperature drop in the O2 tanks and feed lines as the oxidizer expands during consumption, but it may not be enough to provide significant cooling to the fuel tank. Don't quote me on that because I haven't done the math to evaluate the practicality of using oxidizer expansion to keep the fuel supply "liquid". Maybe you could supply enough highly pressurized CO2 to provide a meaningful cooling effect, though. We need to do the math to figure this out.
Offline
Like button can go here
#506 2025-01-13 18:49:41
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I appreciate your patience and hope we could toss some ideas around. I can see that you have some insights that I have missed.
My eye is more on the obtaining of Venus crossing asteroid materials to get started.
Then a diver, that could vacuum up dust and sand.
You are very interested in mining high quality ore veins on the surface, I believe.
For my concept of a diver, coolants in liquid form can allow a dive down to the surface, so I would think in that manner.
Let's say that for the Oxidizer, you have the method of necessary resort, if no other way can be found.
On the fuel side though I am starting to think about a wood gas internal combustion engine.
Here is a video: https://www.bing.com/videos/riverview/r … ORM=VRDGAR
One concept of this is that a diver would run on the gas and liquids produced, and might dump the residue out on the surface, which may contain a fair amount of Carbon. The dumping of the Cabon would be like dropping ballast.
Please remember I am trying to find some ways to do things, I am not seeking a conflict or to annoy you.
Just Rember I am to some extent a "Half-Wit". A jack of many trades. This allows me to cross reference things, but not all attempts produce a value.
Oxidizer is a real problem as you seem to have figured out. I have considered Sulfuric Acid. This might be compatible with a ceramic engine, I would hope. Apparently, it is not a very good Oxidizer without supplemental heat. But on the surface of Venus, we have supplemental heat. But of course keeping it liquid will be a challenge. Handling hot H2SO4 will also be a massive challenge.
But I may be on the wrong track, with Sulfuric Acid. It is my impression that it can deal with Carbon if heat is added, and I expect heat to be added on the surface of Venus.
It will not be as bad to keep as a liquid on the surface of Venus as would LOX be, but it would be a problem.
The divers I have thought about might be able to fly over a funnel an discharge the liquid into a storage tank.
I am not going to tell you that I have any high level of assurance that H2SO4 would be a suitable Oxidizer on the surface of Venus. I probably need guidance on that. It is quite available at altitudes on Venus.
Nitric Acid might be something, but it is said to be nasty.
But I am glad you called me on this. I think I have had an insight about Sulfuric Acid in the clouds. Apparently it may be supercooled.
https://www.sciencedirect.com/science/a … 3323001198
Quote:
•
Venus cloud droplets are supercooled and so they remain liquid and will never freeze anywhere in the cloud deck.
I am not sure what that means. Does it suggest that if we provided nucleation points we could get the materials to freeze and fall as precipitation?
A quick look indicates that cloud seeding on Earth works if there is super cooled water in the clouds.
So, while I may have interfered with your ideas for Oxidizers, we may have a trick to alter the nature of Venus.
If we seed the clouds, we may introduce convection. If we do that then H2SO4 will move to altitudes where it will be heated so much that it will decompose into H20 and SO2 or SO3. (I hope).
Where Venus inhibits convection, if divers could bring up dust and sand, that could be processed and possibly the tailings, (Waste) could be used to seed clouds, if it were desired to try to reduce the acid nature of Venus.
Now, I withdraw and confess that you may indeed have the correct notions for surface operations, and I interfered with your work.
But I think it may have been worth it to learn some new things.
My apologies.
Please retake the wheel.
Ending Pending ![]()
Last edited by Void (2025-01-13 19:22:47)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#507 2025-01-13 22:37:07
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,483
Re: Venus
Caterpillar 797B Mining Truck Fabrication and Assembly Video:
Caterpillar Manufacturing: Inside the Factories Building Behemoths | FD Engineering
Offline
Like button can go here
#508 2025-01-14 10:31:37
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
That is very impressive. We had similar trucks in the mines, but I did not know where they came from.
My comments now are not for disputing any of the work you are doing.
But I want to suggest how it may be practical to store and use cool or cold fluids on the surface of Venus.
Coffee...........
OK, I got ahold of this calculator and may or may not be using it correctly. For water it seems to indicate 92 bar at a temperature of 540 F. (282 C).
And I am goofing here a little bit, but the bigger your tank the better, similar to how large animals handle cold better than small ones for amount of surface area per volume.
So, if we have a well insulated depression in the ground on Venus, with a roof over it, and a drain that can allow a water drop from an aircraft, to quickly enter the tank, we might hole the water for a certain period of time that could be useful.
Maybe the aircraft is filled with temperate water as ballast. It travels downward to the surface, hooks a load of ore or processed materials and dumps a large volume of water out of itself to become light enough to ascend. Chemically powered engines might work for it or steam powered engines. As if traveled down the water in its tanks might heat up, and if it had some water left over to ascend with the water could boil to turn an engine all the way up, with the boiling point dropping as it moved up to lower pressures.
Sulfuric Acid would be somewhat similar, but it decomposes at heating.
This looks to be above my pay grade, but I will try: https://chemistry.stackexchange.com/que … to-heating Quote:
The decomposition of H2SO4
to H2O
and SO3
is predominant between 400
and 700 K
. The formation of a small amount of gaseous sulfuric acid can be observed. Above 673 K
, the equilibrium constant of the reaction R1 becomes higher than 1 and increases rapidly.H2SO4↽−−⇀H2O+SO3(R1)
So, I will convert temperatures: 400 K > 126.85 C and 700 K > 426.85 C
Average temperature at the surface of Venus: https://www.space.com/18526-venus-temperature.html
Quote:
The average temperature on Venus is 864 degrees Fahrenheit (462 degrees Celsius). Temperature changes slightly traveling through the atmosphere, growing cooler farther away from the surface. Lead would melt on the surface of the planet, where the temperature is around 872 F (467 C).
Temperatures are cooler in the upper atmosphere, ranging from (minus 43 C) to (minus 173 C).
Just now, I am thinking this could be workable.
Drop the Sulfuric Acid load into a tank from an aircraft, then slow cook the SO3 (Trioxide) out of it and have a leftover of hot water.
I don't know if it would be hard to do liquid CO2, I suspect it is a possibility.
And then we might consider LOX and Liquid Nitrogen, but those will be hard.
Fuels might be interesting, but we have enough on our plates for now as far as I am concerned. Carbon or a very heavy Tar/Oil might be considered.
Sulfuric Acid: https://en.wikipedia.org/wiki/Sulfuric_acid
Quote:
Boiling point 337[1] °C (639 °F; 610 K)
When sulfuric acid is above 300 °C (572 °F; 573 K), it gradually decomposes to SO3 + H2O
So, as it would shed SO3, I am inclined to think that would be a sort of boil off that would temporarily keep the water from boiling off. But I am not sure.
One misgiving I have is the very dry dense air at the surface of Venus will probably try to suck the moisture out of the tanks by dissolving it so that problem would need handling.
I am going to be traveling so I may not be in touch as much for a bit.
Ending Pending ![]()
Last edited by Void (2025-01-14 11:06:49)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#509 2025-01-15 01:30:41
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,483
Re: Venus
Void,
If there is a lot of elemental Sulfur readily available on the surface of Venus, then Sulfur can be combusted with pure O2 to yield about 9.16MJ/kg of Sulfur. That's 3.58X lower energy density than combusting pure Carbon with O2, but elemental Sulfur would remain liquid and then transition to a vapor in the combustion chamber, making it ideal for burning. Sulfur boils around 445C, but it could be stored and kept liquid under modest pressure. If most of the Sulfur dioxide gas could somehow be retained after combustion, then it could become a viable recyclable energy store. SO2 density is pretty high when compressed to 500bar or beyond, just as CO2 becomes very dense at such pressures.
2SO2 + 2CO → 2S + 2CO2
Maybe we need to use some mix of Carbon and Sulfur together as part of an endless recycling of the combustion products?
Just a thought.
Offline
Like button can go here
#510 2025-01-15 12:20:49
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
That sounds like it has some potential.
Sulfur has been found on the Moon and Mars, I believe.
I think some games may be played with chemistry on Venus. But step #1 is to be sure at a high level that life does or does not exist on Venus. I am thinking of the clouds, so unlike Mars, that could be tested fairly well, I think.
If it is confirmed that life does not exist, then I suggest we consider increasing the greenhouse effect on Venus.
For two reasons: 1) To give UV protection and so then to convert H2SO4 to SO3 or SO2, and H20. 2) To expand the atmosphere, so that it could be extracted to orbit from a higher position in the gravity well of Venus.
Here is a possible tool: https://www.colorado.edu/today/2010/06/ … lder-study Quote:
CU Boulder Today Early Earth Haze Likely Provided Ultraviolet Shield for Planet, Says New CU-Boulder Study
Published:6/3/20106/3/2010
So, in any case, we have the needed molecules to make a haze like Titan has. Methane for instance would apparently be converted into other things such as
At the hot base of the cloud deck on Venus, H2S04 decays into H20 and SO3. When SO2 and water are influenced by UV light then H2S04 is created.
Here is a related article in another topic about Earth: https://newmars.com/forums/viewtopic.ph … 48#p228948
Quote:
I find that this history of Earth is important.
https://www.msn.com/en-us/news/technolo … d31b&ei=18
Quote:How life began on Earth: Modeling the ancient atmosphere
Story by Science X staff • 2mo • 2 min readImage Quote:
Quote:Diagram of the atmospheric evolution of Earth's ancient atmosphere estimated by this study. Credit: Astrobiology (2024). DOI: 10.1089/ast.2024.0048
Quote:
The calculation reveals that most hydrogen was lost to space and that hydrocarbons like acetylene (produced from methane) shielded UV radiation. This inhibition of UV radiation significantly reduced the breakdown of water vapor and subsequent oxidation of methane, thus enhancing the production of organics.
I find the idea that Acetylene may have given protection from UV radiation. I wonder what sort of a greenhouse gas that gas could be for Mars.
Three possible ways to warm Mars would be greenhouse gasses, particles, and high water ice/vapor clouds.
If in addition Acetylene would offer UV protection, perhaps hardy Arctic, Alpine, and Antarctic life might be supported on the planet Mars.
Ending Pending
So, we have a primary conditions on Venus. Either it is having it's H2 replenished, or we just accidentally viewed it when it had the last of it's H2 in the clouds. I think the more probable idea is that it's H2 is replenished from some reservoirs, even if some H2 is carried away by the solar wind.
And so the two reservoirs that I can think of are either internal to Venus or external to Venus. External might be the solar wind or impactors.
So, the chemicals exist to make a haze like that of Titan. Making Methane might lead to the creation of Acetylene.
I guess the hope would be a haze layer well above the H2SO4 and H20 cloud layers. No guarantee for that, just a possibility.
If the atmosphere of Venus swells up then it's top will be further up in the gravity well of Venus. This might be assistive in the ability to collect atmosphere to put into O'Neill type habitats made of captured asteroid materials or materials mined from Venue.
Inside of the atmosphere of Venus then it may become less acid. Also if large floating structures were created, then Starship SSTO could drop down to say 5 or 10 bars depth, the have a very slow terminal velocity or even float.
Then the elevator might move the starship up to say the .33 bar level to launch it to space again, maybe SSTO, as the gravity of Venus is less than that of Earth.
If you are making Methane and Oxygen for Starship, then you perhaps could make Methane to make a haze on Venus.
Ending Pending ![]()
Last edited by Void (2025-01-15 12:50:18)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#511 2025-01-15 20:28:18
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
Actually this video was simply entertaining to me. But, in the previous post I suggested swelling up the atmosphere of Venus, so that the atmosphere would experience lower gravity. So, then I suppose I think that this might allow a velo atmospheric system to work better.
I don't know if an "Electret" could be helpful, but I am looking for helpful things for such an intention.
https://www.bing.com/videos/riverview/r … &FORM=VIRE Quote:
Creating an Exotic Material With A Permanent Static Charge (Electret)
YouTube
Plasma Channel
471.1K views
1 month ago
So, apparently it can attract matter. While I am interested in collecting atmospheric molecules, it also can work to collect dust, as perhaps from an asteroid or the Moon, I suppose.
I dust is not all of the same materials, then could you spin a Electret and separate types of grains of dust, where they may have different responses to the electrical field, and also have different material densities for inertia. Using electrostatic cling to separate substances using also a mechanical rotation.
A flow of a fluid, perhaps a gas of some type at some pressure might also be included in the process.
Just entertainment, no strong belief of accomplishment beyond that.
Ending Pending ![]()
Last edited by Void (2025-01-15 20:33:54)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#512 2025-01-16 11:36:22
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
While I regret deviating from the work of kdb512 here about motors for Venus, he has already contributed much that is of interest.
I have deviated into methods of terraform of Venus using hydrocarbons as greenhouse effect and to modify UV's influence on the production of Sulfuric Acid.
I have already stated and affirmed that if Venus has life, then we can do without Venus. I think it is more improbable that Venus has life, but it is required to honestly look for it.
Without the existence of life on Venus, I think it can be supposed that swelling the atmosphere of Venus could be of value. Historically I had read of slamming the planet with an asteroid so that the atmosphere would swell up and be blown away by the solar wind. Then, supposedly the planet would be useful in 10,000 years or so. I do not like that for the time duration necessary, and for the fact that I want to harvest the atmosphere, not throw it away.
If we use greenhouse gasses to swell up the atmosphere of Venus, then it seems we may also reduce the UV conveyed to lower parts of the atmosphere, and so tip the balance to allow less production of Sulfuric Acid, and promote the retention of H20 and SO3. This may make the environment more machine friendly for cloud cities.
So, we can use a tool that is intended for Mars, to heat up a planet that is already very hot, but might hope to have useful results from that.
But now there seems to be a new tool for heating up Mars: https://www.japantimes.co.jp/news/2024/ … p-glitter/
Quote:
WORLD / Science & Health
Scientists propose warming up Mars by using heat-trapping 'glitter'
This tool also might work to swell up the atmosphere of Venus.
If the Atmosphere of Venus were considered a resource, then what it has to offer in declining magnitudes are Oxygen, Carbon, Nitrogen, Sulfuric Acid, Water, and some smaller things.
The heating methods I have described might not heat up the Exosphere of Venus, so may not promote air loss by heating, but it might elevate the Exosphere and make it more reachable by the solar wind.
Raising the upper atmosphere would reduce the gravitation experienced by the upper atmosphere. I am interested in atmospheric mining, so this might be useful. From my point of view this raises the orbital energy's of atmospheric atoms, even if they are only on suborbital pathways.
But my desire is to harvest the atmosphere, not so much to let the solar wind drag it away. So a magnetic field might be imposed to control the solar winds access to the swollen atmosphere. Of course this sounds expensive, but if the value of the product justifies it then the cost of a magnetic field might be tolerated. I would think that a leaky magnetic field would be preferred, to allow the solar wind to push itself into the atmosphere and mix with it but not carry it away.
I don't know how possible that is.
A magnetic field might also cause gasses that leak from orbital habitats to drop into the atmosphere of Venus, so this would be a form of conservation.
It orbit it may be possible to build structure in part with Carbon from the atmosphere of Venus. I have also suggested various ways to bring stony asteroid materials into the orbit of Venus, from small asteroids that cross orbits with the terrestrial planets. These materials could also contribute to habitats in orbit, and floating structures in the atmosphere of Venus.
kdb512, myself, and others have posted about various ways to mine the surface of Venus. These materials may also make a contribution to both floating and orbital structures.
Nitrogen and Oxygen from the atmosphere of Venus may be used to fill orbital structures along with Oxygen from Stony Asteroid materials.
I am interested in Very Low Orbit spacecraft of various kinds to lift atmosphere up to those orbital structures.
Here is an article about that for Earth. It may be adaptable to Venus: https://en.wikipedia.org/wiki/Very_low_Earth_orbit
Quote:
Very low Earth orbit
I also think that tether technology may be supportive of scooping atmosphere from Venus up to orbital structures.
While some people support the idea of cloud cities, I prefer orbital structure. But cloud cities are fine, but I think maybe more, robots would inhabit those for the most part with just a few humans. Orbital habitats would be bathed in a large amount of solar energy available. If they would be built substantial enough to support humans this could be a very good contribution to a solar system wide habitation process.
Ending Pending ![]()
Last edited by Void (2025-01-16 12:08:00)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#513 2025-04-06 21:45:28
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I have an interest in Venus. Mars and the Moon probably need to come first, but I think Venus and the asteroids as partners will be of significant value.
In this topic about the Moon: https://newmars.com/forums/viewtopic.ph … 12#p230812
materials including and between posts #226 to #232 may partially apply.
But there are other things that may apply:
Fusion Rockets: https://www.msn.com/en-us/news/technolo … 390a&ei=17
Quote:
Startup Says Its Nuclear Fusion Rocket Could Cut Time to Mars in Half
Story by Victor Tangermann • 2h •
3 min read
And such can be of importance throughout the solar system.
And also, something of value might come from this:
https://www.msn.com/en-ca/news/technolo … r-AA1C94No
Quote:
These Space Sails, Built in 24 Hours, Could Enable Fast Interplanetary Missions!
Story by Arezki Amiri • 4d •
3 min read
With robotics, I anticipate that Venus will become a very rich world. I expect that mass will be delivered to the orbits and atmosphere of Venus by these means. The materials could come in part from our Moon, but also the asteroids.
Ending Pending ![]()
I am of course interested in any world with a significant atmosphere, by moving needed materials to locations desired, using various methods, including aerobraking, other worlds can be developed. Among these I include Mars as an early one and Titan as a later one.
Ending Pending ![]()
Last edited by Void (2025-04-06 21:59:21)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#514 2025-04-07 20:39:31
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
So, I have tried to have a plan(s), and those are not necessarily the best plans possible ever.
But how I see it is eventually robotic labor productivity does not become infinite but tries to become infinite.
The Asteroids provide an enormous amount of mass which may be transferable to the orbits of Venus or to the clouds of Venus. The easy stuff is more the stony stuff, the further away stuff is leaning towards things like Carbon, Hydrogen, and even Nitrogen.
But Venus has Carbon, Nitrogen, and even Hydrogen.
Given various means of propulsion, asteroid materials could be put on an intercept of Venus more easily than to propulsively rendezvous with Venus. So, Aerobraking could be a shortcut.
In this post: https://newmars.com/forums/viewtopic.ph … 28#p230928
I suggest a sandwich heat shield:
Ablative+Stainless Steel+Ceramic Cloth+ Aluminum+Ceramic Cloth+Aluminum Foil.
Here is a notion:
So, my hope is to be able to use the heat shield as a mirror in flight for concentrating solar energy, so an aluminum foil layer, starting on the concave side.
Next Ceramic or Fiberglass Cloth
Next Thick Aluminum
Next Ceramic or Fiberglass cloth
Next Stainless Steel
On the outside convex side ablative materials.
Using quite a few tricks to manage the heat which occurs when air braking occurs.
And in thes post I suggest a method to try to use Oxygen as a heat shield coolant: https://newmars.com/forums/viewtopic.ph … 20#p230920
Quote:
In this case the Oxygen has to be warmed to a gas to be ejected on the leeward side of the heat shield in hopes of cooling it down. Of course, Oxygen is corrosive, but perhaps in low density and not too hot, it can be tolerated. The Ceramic cloth is likely to be the most tolerant of this.
The emission of the dominantly Oxygen plume mixed with the results of a smaller combustion process, will also help to steer the apparatus, as the engine(s) should be able to throttle/gimbal.
Here is a list of Venus crossing and grazing asteroids: https://en.wikipedia.org/wiki/List_of_V … or_planets
They are generally small and rocky in nature. They may have implanted Hydrogen from the solar wind in some of their materials.
Oxygen by far is the greater source of a possible fluid coolant from these. Oxygen of course is nasty in many ways to use for cooling, but I hope I have found a way.
While we can hope for methods of propulsion to get this apparatus to cross the orbit of Venus, such as Nuclear of some kind, solar at these distances from the sun seems to have some merit.
In that case Magdrive or Neumann Drive or solar sails may be options.
It would not be impossible to imagine working with asteroids that were further from the sun, but I thought to start with these.
This sort of heat shield, setup has some resemblance to the parachute, sort of backwards.
The primary objective is to get the heat shield sandwich into a Venus orbit though, so the spacecraft is assistive to the air brake device, not the other way around as in a parachute.
So, a formulation of surface area, thickness, and components of the sandwich are going to require various recipes as this is unfamiliar territory.
But as I have said, robots will reach towards an infinite labor supply, and the sunlight and nuclear can provide a great deal of energy.
So, using this method the orbits and clouds of Venus could be supplied with useful materials.
Granted someday it may be practical to mine Venus itself, but it may remain easier to supply the orbits from asteroid materials even then.
So, I think Venus could become quite a valuable sub-part of a solar civilization.
Ending Pending ![]()
Last edited by Void (2025-04-07 21:07:25)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#515 2025-04-08 09:19:50
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I have been more of a fan of habitats in the orbits of Venus than in the clouds of Venus, primarily due to the Sulfuric Acid.
I can think of notions to alter Venus, as far as its atmosphere goes, but the first thing is to make sure that the clouds do not contain life. If they do, then I feel alteration of Venus can likely be considered to be a wrong thing to do.
I will consider it very likely that no life based on Silicon, exists on Venus. If it did, then it should also be likely in the lava of Earth. We have not encountered anything like that on Earth.
Things that could be altered for Venus could be the level of acid, and the height of the atmosphere.
If we could reduce the UV striking Sulfur Oxides and water vapor, then we could inhibit the production of Sulfuric Acid. At the base of the cloud deck Sulfuric Acid decomposes into Sulfur Oxides and Water Vapor.
It might be possible to create an Ozone layer, but I have no certainty that that could be done.
Methane might be helpful; it is speculated that it had benefits for the Earth early in its history.
https://www.livescience.com/10668-thick … earth.html
Quote:
A thick organic haze cloaked early Earth several billion years ago and may have kept the planet from freezing over, protecting primordial life from the damaging effects of the sun's ultraviolet rays, a new study suggests.
The haze, made from methane and nitrogen chemistry in the upper atmosphere, would have been analogous to the cloudy curtain hovering above Saturn's largest moon, Titan, the researchers say.
The results help solve a longstanding mystery called the faint young sun paradox: While geological evidence suggests early Earth was ice-free, climate models haven't been able to get the planet warm enough for such a wet, toasty world.
This might also explain how Mars worked a long time ago.
I suspect that it might be possible to create Methane at a rate, faster than the Venus environment could destroy it. It is relatively a good lifting gas, particularly in the dominantly CO2 atmosphere of Venus. Hydrogen and Helium are better lifting gasses, but Methane is what might do the good desired.
A hope would be that it could dwell in the higher atmosphere above the major amount of Sulfuric Acid and Sulfur Oxides, and water vapor. Water Vapor would be a lifting gas in this environment also, but of course it will condense into snow and rain at higher altitudes.
If a haze of tholin would develop, in the high sky, it may in part block sunlight and may be part of reducing UV light. I am not sure if this would warm or cool Venus. The Methane might likely warm the planet.
But Sulfur compounds might cool it. This article says it needs to be done correctly, or it may warm the planet rather than cool it: https://www.sciencealert.com/injecting- … rous-risks Quote:
New research warns that if we inject sulfate particles into the atmosphere to attempt to reflect sunlight and mimic the cooling effects of volcanic eruptions and they don't end up in the right position, they could cause further warming and even worse climate anomalies than burning greenhouse gases as usual.
And I wonder if such an injection would interfere with the Methane and Tholin that may be desired.
Dust might be used as a cooling agent.
But specially made dust might warm a planet it seems: https://news.uchicago.edu/story/scienti … -warm-mars
Quote:
In a groundbreaking study published Aug. 7 in Science Advances, researchers from the University of Chicago, Northwestern University, and the University of Central Florida have proposed a revolutionary approach towards terraforming Mars. This new method, using engineered dust particles released to the atmosphere, could potentially warm the Red Planet by more than 50 degrees Fahrenheit, to temperatures suitable for microbial life—a crucial first step towards making Mars habitable.
So, some tools. In my notion of ideal, if the planet is lifeless, then reducing the amount of Sulfuric Acid would be a good thing.
Also, unlike most others who want to deal with Venus, I want to be able to extract resources from Venus. This might be facilitated by an ideal atmospheric height. Temperature changes could alter the atmospheric height.
The changes I have suggested in this post, might make the environment of Venus more compatible with machinery in floating habitats.
So, if I were an inhabitant of Venus, I might be divided on the idea of living in the clouds or in orbit. In orbit you need additional protections for radiation. If you have the materials, then that should not be an impossible problem to solve.
One thing I have wondered about is to create an orbiting magnetic field, that grazes the atmosphere of Venus. My hope is that the magnetic field could capture ionized gasses into itself.
And then in further steps that that mass could be recovered to orbit.
But the process of capturing mass in that manner would drag the orbiting object down back into the atmosphere, so some kind of propulsion is needed to help lift the process to orbit.
But maybe Venus has a lot of promise as for hosting a very large population in the future.
Ending Pending ![]()
Last edited by Void (2025-04-08 10:04:28)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#516 2025-04-09 14:33:00
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
A thing I have been thinking on today, is if floating cities in the clouds of Venus could in part be floated on chambers filled with Methane instead of Nitrogen or Air.
https://brainly.com/question/30357778
Quote:
The specific gravity of methane is 0.554 and the specific gravity of propane is 1.52.
Almost double the lift of air. That could be helpful in the clouds of Venus. An apparatus could float higher in the sky, I think.
That would not necessarily be wasted volume, because robots of various types could work inside such volumes.
And I would not worry too much about the Methane leaking into the atmosphere of Venus, as I think that Methane and tholins might modify the environment of Venus. My hope is that they would block UV light and allow the Sulfuric Acid to decompose into Sulfur Oxides and water vapor.
Two energy sources for Venus in the clouds could be solar and differential wind. Differential wind might work, if you have sort of a sea anchor that hangs down and experiences a different wind condition than a windmill.
Ending Pending ![]()
Last edited by Void (2025-04-09 14:45:33)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#517 2025-04-09 19:44:45
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
This article has some ideas for a cloud city: https://www.pinterest.com/pin/359865826453439334/
I still tilt towards orbital habitation for humans, with perhaps workers in the clouds.
I would expect the cloud cities to mostly be inhabited by appropriate robots. And that would reduce the life support required quite a bit.
Also, robots could work in a Methane atmosphere, which would provide more flotation than air or Nitrogen.
With very few humans in the cloud "Cities", no need for food production and water for the larger part, except for a very small number of humans.
At first mining the atmosphere might be the primary activity. Starship like vehicles might lift Methane to orbit. Of course, the ability to work with sulfuric acid will be important as that is where the Hydrogen would come from. Also, some methods are needed to make spacecraft tolerant to the environment.
And attempt to harvest atmosphere from orbit might work out. Tethers might work, maybe a magnetic field that captures ionized atmospheric gasses. Ideally Oxygen, Hydrogen, Helium, Nitrogen, CO2. But I do not know if a method can get all of them.
If asteroid materials can aerobrake to orbit of Venus, then large habitat structures could be assembled in orbit, and Venus in some way or another can provide the atmospheric resources, such as Nitrogen, water, Carbon, Oxygen.
As for Ships, entering the atmosphere and launching to orbit, beyond the acid problem, I think it is a pretty good game. To "Land", you would just dive down until you floated from air in the ship. You would not need an engine burn.
Then some type of very large aircraft might retrieve your ship and bring it to a launch platform. And 90% gravity would be favorable, for SSTO, perhaps.
Ideally if Methane could alter the acid situation of Venus in the long term, then the environment would be even better.
Ending Pending ![]()
Last edited by Void (2025-04-09 19:57:43)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#518 2025-06-25 12:44:36
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
New asteroids in association with Venus. That interests me: https://www.msn.com/en-us/news/technolo … r-AA1HkVhe
Quote:
A hidden asteroid family may share Venus' orbit: 'It's like discovering a continent you didn't know existed'
Story by Sharmila Kuthunur • 1d •
3 min read
Quote:
In a study published earlier this year in the journal Icarus, Carruba's team analyzed the orbital evolution of the 20 known Venus co-orbital asteroids. Their simulations showed that three of these objects — each measuring between 1,000 and 1,300 feet (300 to 400 meters) across — could eventually pass within about 46,500 miles (74,800 kilometers) of Earth's orbit. In some cases, this gradual shift onto a near-Earth trajectory could take up to 12,000 years.
So, that is interesting to me.
Ending Pending ![]()
Last edited by Void (2025-06-25 12:48:59)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#519 2025-06-26 12:29:30
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
In the previous post it is said that there are found many more Venus crossing asteroids. I feel this could help jump start a settlement process for Venus.
But in another topic Ascender has caused me to ask if it could be appropriate for Venus: https://newmars.com/forums/viewtopic.ph … 93#p232393
Quote:
At this moment where faith in rockets may be challenged, I guess this is interesting to look at again: "John Powell, This Airship Might Change How We Reach Space Forever!" https://www.youtube.com/watch?v=fdfTCBRwijI
Quote:This Airship Might Change How We Reach Space Forever!
John Powell
15.2K subscriber
What has my attention is that the Venus atmosphere is swollen from the heating of lower layers, and the gravitation is ~90% that of Earth. Most layers of the atmosphere are dominated by CO2, perhaps not the highest layers though?
So, if the atmosphere is swollen??? (The CO2 will weigh it down more, the gravitation is ~90% that of Earth, the Atmosphere is extremely heated). So, the Ascender might find it easier to get to orbit from a high flotation level.
I would also alter their Ascender to be powered by microwaves rather than solar.
Microwaves could perhaps supply many times more power than the solar panels they currently envision. Orbital Solar Power could beam the energy to the rectennas built into the structure of the Ascender. So, it may have more electrical power. Gravity of Venus being lower, it may be that more mass could be devoted to engines, and propellants.
Coffee.............
If Hydrogen Gas is to be the flotation gas for Ascender, then with microwaves it might be heated up as well, so your flotation may be both of a Lighter Than Air Gas, and a heating of the gas to assist flotation. This would then end up with less Hydrogen mass in the Ascender.
Space Elevators will not work for Venus, but perhaps some sort of Tether might. So, the Ascender might only need to achieve a Very Low Orbit, and its cargo might be grabbed with a Rotovator. After that being in a VELO orbit, the Ascender will naturally settle back down towards the "Sky Station" At a high altitude.
I need to review the video again to get there correct terminology.
I will say though that I see the possibility of multiple levels to even access the surface of Venus.
1) Orbital Power Plants.
2) Rotovators.
3) Ascender.
4) Sky Station.
5) Transit Zepplin.
6) Cloud Cities.
7) Ballasted Aircraft.
8) Ground Power Plants.
The #7 Ballasted Aircraft would be filled with Sulfuric Acid Liquid in the clouds, and then released to plunge towards the surface. They would have a lighter than air feature in them so that as they approached the surface they would become more and more buoyant. Then very near the surface they would dump their Sulfuric Acid Liquid into a bin covered with a glass grill. Sort of resembling vomiting, perhaps. The Ballasted Aircraft might be able to hook a load onto itself and then ascend into the Sky as it had disgorged its ballast.
The Sulfuric Acid would go into the bin and heat up and emit it's SO2 and SO3, leaving rather hot water.
The water would be used to power robots by steam power. I presume that the water could boil and that the output would be very strong for power, supercritical. (Am I wrong? Will the pressure at the surface of Venus be too high?)
If levels 1-8 could be implemented on Venus, then it would be possible to extract minerals from the surface of Venus and lift them all the way up to orbit to make things like power satellites.
But to get the thing started Venus crossing asteroids could prove useful.
Ending Pending ![]()
Last edited by Void (2025-06-26 12:57:33)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#520 2025-06-26 20:41:20
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
(th) has had a comment for me to respond to: https://newmars.com/forums/viewtopic.ph … 47#p232447
I believe I don't know as much about rectenna's as I might want to. But I have supposed that it might be a bit like chicken wire? Well it is certainly more complex than that, but if it could be incorporated into the walls of the Ascender, and also collect power, perhaps that could work well.
On Earth the microwave source could be on the ground. If you sent that intense of a microwave to the ground from orbit it might cook people.
On Venus I expect it would be in orbit. If it could deliver 10 times as much power as solar would to the Ascender rectenna, then perhaps it could run a VASIMR engine. https://en.wikipedia.org/wiki/Variable_ … sma_Rocket
It could possible expel Nitrogen or CO2 from the atmosphere of Venus.
But maybe the Chemical/Electric engines spoken of could be made to work.
People inside of the Ascender would need something like a Faraday Cage to be protected from the Microwaves???
At least I think that is barking up the right tree, more or less.
As for the Hydrogen flotation gas, perhaps that could be superheated with a different frequency of microwaves, to reduce its mass and increase flotation as the device would rise higher in the atmosphere. But you would have to not damage the Ascender walls or other parts. That might be possible if the Hydrogen was to low density that it did not overheat the Ascender walls.
Maybe I have it half right?
As for Space Elevators, Venus could have Rotovators, or Skyhooks, but not a ground anchored Space Elevator. At least that is what I have read.
Ending Pending ![]()
Last edited by Void (2025-06-26 20:55:16)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#521 2025-06-27 09:26:43
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
Using such a "Aircraft" as a signal repeater may make sense.
If data could also be received by the rectenna, and then if the device can transmit data.
Perhaps a device to hover over a city.
Ending Pending ![]()
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#522 2025-06-27 13:23:34
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
In the case of Venus, further manipulations that might be considered are;
1) Add Methane to the atmosphere, and indirectly, perhaps, Tholen's.
2) Use particles to warm the planet that have been proposed for Mars: https://www.impactlab.com/2024/08/17/wa … e-thought/
3) Add a Magnetic Field to L1, perhaps, as has been proposed for Mars.
1) Adding Methane would be a greenhouse effect, but the creation of Tholen's may cool the planet. But both of these may block UV light, changing the chemistry of the planet. This may inhibit the synthesis of Sulfuric Acid but promote the creation of Sulfur Oxides and water vapor.
2) This one will heat the planet and cause it to swell up.
3) This may help avoid atmospheric losses to the solar wind.
Historically, one proposed method to terraform Venus would be to slam an asteroid into the planet to swell the atmosphere, and make the atmosphere float off, and to then have a thinner atmosphere in 1000 to 10,000 years.
I do not approve of that, but if we could swell up the atmosphere of Venus, then something like Ascender might work better. If the atmosphere swelled out to the limits of the Hill Sphere of Venus, then the atmosphere would likely start to be pulled off by the solar wind. However, if we needed to we could put a protective magnetic field into L1 for Venus.
While I am hoping that Ascender could travel to the edge of the edge Hill Sphere of Venus where gravity will be much less limiting, also I am hoping to "Harvest" the Atmosphere of Venus, as a raw material to turn into resources for the Solar System.
Things that might make us want to limit this process would be we don't want to make the surface too hot for robots, and we may not want to swell the atmosphere all the way out to the edge of the Hill Sphere of Venus. Too swollen would be if the atmosphere starts leaking off into space too much.
Given Robots and this process it may be possible to canister the atmosphere of Venus and deliver it to Mars and perhaps Jovian Moons.
But if we find life in the clouds of Venus, I will say we will not do this.
Ending Pending ![]()
Last edited by Void (2025-06-27 13:45:54)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#523 2025-06-29 11:23:52
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I want to speculate on things that I desire, but cannot in a substantial way, affirm good methods to achieve these desires. But this can have value as it indicates where we could look to find ways it could be done.
Supposing that the raw materials of Venus could be to some extent converted to resources. The Atmosphere of Venus might have been swollen a bit by a greater heating, and a artificial magnetic field imposed to reduce the loss of gasses being swept away by the solar wind.
The atmosphere of Venus would in itself be a raw materials source. Eventually it might become possible to mine the surface of Venus even if it is very hot. But before that asteroid materials could be brought to Venus, and possibly materials from the Planet Mercury.
A primary reason I want a magnetic field for Venus is that I view the construction of orbital habitats as being of a good potential to create wealth. But such structures will leak. I want the leakage to go back into the atmosphere of Venus rather than to be pulled away by the solar wind.
Nitrogen is something that Venus has that has some value. Carbon Dioxide could become a chemical method of propulsion. At the very least CO and O2 might be worth the trouble in orbital space. And possibly Carbon itself could be preheated with solar energy and then burned against Oxygen.
But Carbon can also be a construction material. Although not as good as water, Carbon and Dry Ice might be suitable to be radiation protection. Of course, in the orbit of Venus maintaining Dry Ice as to not evaporate could be a challenge, but I think not impossible.
Of course a method to extract the materials from the atmosphere of Venus would be needed. Ascender, Rotavators, and rockets might be possible in the future.
A thing I have in mind is a conveyor method to move atmosphere from Venus to other worlds like Mars.
If regolith derived materials could be used along with atmosphere from Venus, then these habitats could be moved at a slow speed to eventually orbit Mars.
If a protective magnetic field were installed around Mars, then when these habitats leak the gasses might then accumulate into the atmosphere of Mars and then be available to recycle.
A multihull method could help to conserve N2 and O2, on a long travel from Venus to Mars Orbits: 
Pumping methods would return leaked gasses to the appropriate shell as they did try to leak out into the vacuum of Space. A bit of CO2 in the outermost compartment might help to keep leakage into space at a minimum. Ideally the outer compartment would not be pressurized to the point of viscous flow, so molecular flow would dominate.
I recall reading that a generation ship sent to the nearest star would be completely drained of atmosphere by the time it arrived at that star. And so being familiar with high and low vacuum systems, I have devised this method of trying to postpone that event, perhaps to allow the retention of atmosphere until arriving, and possibly longer.
The core chamber in the center is 1 bar N2/O2. Actually, it might be up to 5 bars, I think. We hope for it to arrive at Mars with an excess of atmosphere, especially Nitrogen.
Then in this plan slow leakage might being the highest pressure within, down to 1/2 Bar N2/02 mix, with that actually being sufficient for human usage, I believe.
To get regolith for this at Venus, air braking might be used from solar orbing objects. Alternately mining the surface of Venus might become possible.
As the multihull structures would in effect be multi-generation ships, they do not have to travel to Mars in any hurry.
They might be able to sail on Photons, or the Solar wind. Alternately the Neumann Drive could run on Carbon. Or an Oxygen Mass Driver might be used.
But if it takes centuries to get to Mars, that should not be a concern. They would be worlds in themselves, within a solar system full of such worlds.
So, some partial tenuous solutions to advanced terraform methods that might be used.
Obviously Titan offers a chance at something like this as well, for having lots of Nitrogen and Methane and water ice.
Ending Pending ![]()
Last edited by Void (2025-06-29 12:07:09)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#524 2025-06-30 08:15:23
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
I have an eye on space rocks that penetrate into the inner solar system.
It seems that Tesla will be building factories that build Optimus Robots. This begins to approach: https://en.wikipedia.org/wiki/Von_Neumann_machine
I am thinking that any asteroid that penetrates into the inner solar system could eventually be utilized to build structure to airbrake into an orbit of Venus.
There are so many methods of propulsion that are emerging. Most are discarded in the minds to space thinkers, but that is largely because they are Slow-Mo methods. But if habitats are constructed from inner system asteroids and then at Venus to pick up many amounts of volatile materials, these then can expand the domain of humans and their robots.
The only piece that is missing is large quantities of Hydrogen; Venus can only give a little. So, desert biomes perhaps in these things at that point. But then traveling out to Mars and maybe the Asteroid Belt to get more water, and bringing an excess of Nitrogen and Carbon with them.
Then perhaps parking in Mars orbit. But then maybe in some cases Earth orbit?
With very low labor costs, and new methods of Slow-Mo propulsion I do not think that it is that absurd.
It might turn out to work out.
Ending Pending ![]()
Last edited by Void (2025-06-30 08:26:16)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#525 2025-06-30 17:32:38
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,254
Re: Venus
Looking at #523 post again:
A multihull method could help to conserve N2 and O2, on a long travel from Venus to Mars Orbits:
Pumping methods would return leaked gasses to the appropriate shell as they did try to leak out into the vacuum of Space. A bit of CO2 in the outermost compartment might help to keep leakage into space at a minimum. Ideally the outer compartment would not be pressurized to the point of viscous flow, so molecular flow would dominate.
The outer shell with very low pressure could be flushed with a gas such as CO2 or H20. Intentionally putting some of the flushing gas into the shell, and then with a pump you could pressurize the result either of those gasses could be pressurized to become a liquid, and the other gasses entrained might centrifuge out.
This pumping method might help to pump the flushing gas to a higher pressure: https://shipfever.com/what-is-an-eductor/ Image Quote: 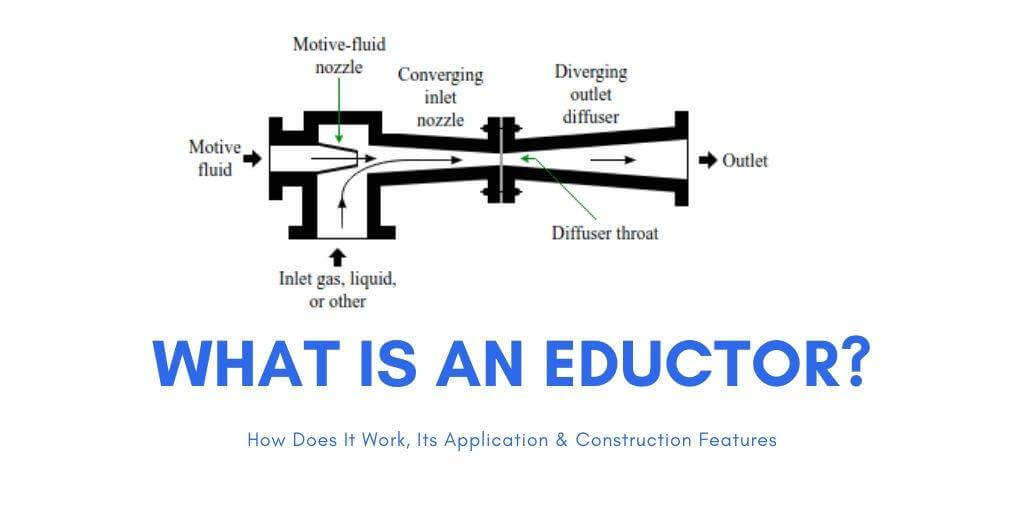
So, then a method to reduce the loss of volatiles over time.
Ending Pending ![]()
Last edited by Void (2025-06-30 17:42:25)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here



