New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2017-04-12 17:25:04
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Iron - the natural fuel for Mars?
I am rather excited at the prospect of developing iron as a fuel. This is a good introduction to the subject:
https://www.mcgill.ca/newsroom/channels … ure-257172
The technology hasn't been perfected yet but looks more than promising. The fact that iron ore is so readily available on the surface of Mars, makes iron fuel a natural choice. But can it deliver on the promise?
What do you think would be the main issues with this technology?
Here are my thoughts:
1. Efficiency. How much energy do we have to put into making the iron powder and into separating out oxygen from either water or the atmosphere in order for the process to work. You can get 6.9 KwHs of energy per Kg of iron according to some figures I looked at. But if it's costing 15 KWHs of energy to get a couple of Kgs of oxygen to complete the combustion process, then this only makes sense as a form of energy storage, like methane.
2. If we terraform Mars, then the oxygen for combustion is going to freely available across the whole planet. At that point iron fuel might really come into its own. Sadly, perhaps not till then.
3. If we develop technologies that allow us to create earth-like spaces with large amounts of vegetation producing oxygen from water, then we might site iron fuel combustion facilities within those spaces, to make use of the earth-like air.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#2 2017-04-13 14:28:14
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Iron - the natural fuel for Mars?
2) Concentrated oxalic acid leaches iron ores and gives Iron (III) oxalate. In a hydrogen atmosphere, iron (III) is reduced to iron (II). After drying, anhydrous iron (II) is destructed by heating into carbon dioxide and iron powder. As metals have vastly different electrode potential, each metal can be electrolyzed directly to the metal or to 2+. metal (2+) oxalates yield metal powder by heating. Note that this heating may be controlled to yield nanometerials of each metal.
Each vehicle would have a tank to keep iron powder and another to keep the iron oxides in solution or powder. When the vehicle is refilled, the two tanks are exchanged; imagine on earth going to garage in exchange of a propane tank and the lead-acid battery.
At an "iron" refinery, the iron oxides are leached with oxalic acid, that itself is produced by a metal catalysted reduction of carbon dioxide. The carbon dioxide itself is either delivered from another factory of Martian atmospheric separation, or produced on site. The water byproduct is recycled in electrolysis to hydrogen and oxygen. Iron (II) oxalate is heated to recycle carbon dioxide and iron powder.
-------------
The following is another aspect of iron powder fuel. Instead of oxidation as energy source to iron (III) or iron (II), iron powder could be oxidized to iron (VI), ferrate in alkali solution. As iron is oxidized from (0) to (VI), more energy is persumably extracted. A drawback is that ferrate(VI) is stable mostly in alkaline solution. A unchecked tank leakage to Martian atmospheric carbon dioxide would slowly turn the solution to less alkaline. Oxygen could then accumulate and either explode itself or trigger a vigorous oxidation reaction with other metal and/or organic parts of the vehicle.
Oxygen phase diagram:
https://encyclopedia.airliquide.com/sites/gas_encyclopedia/files/gas_graph/012_oxygen_p.png
Triple point is 54.36°K (-218.79°C) @ 1.5E-3 bar (1.5 mbar). I don't see oxygen getting cold enough to be liquid at Mars ambient pressure.
At Martian temperature near the surface about (-60oC), solid oxygen sublimes to gas. So an explosion of accumulated oxygen would be possible.
However, an advantage is the ease of manufacture. Alkaline Ferrate (VI) solution in a tank is exchanged at a gas station; imagine exchanging the used propane tank for a new one. The ferrate(VI) is reduced at an "iron refinery factory" by hydrogen to iron at cathode. The cathodic iron is washed and then chiseled into powder if it is not already in powder.
Last edited by knightdepaix (2017-04-13 14:28:56)
Offline
Like button can go here
#3 2017-04-14 03:39:00
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Iron - the natural fuel for Mars?
Is there proof of availability of iron ore deposits in a concentrated form? A large amount of iron distributed thinly over the planet would not be very useful, unless it is dust and can be separated from dunes magnetically without excessive handling of non useful minerals.
Offline
Like button can go here
#4 2017-04-14 04:02:52
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Iron - the natural fuel for Mars?
I think this answer is yes, most definitely:
https://planetarygeomorphology.wordpres … s-on-mars/
Surface deposits are ideal - they can be scooped up by rovers. Remember, we are not talking about iron production in the millions of tonnes as on Earth for the foreseeable future ... more like hundreds or thousands of tonnes. Scoop rovers seem like the best way of collecting the surface deposits. I am guessing, but I would think they could gather 500 kgs in under an hour (under 10 kgs a minute, or maybe three scoops of 3-4 Kgs every 20 seconds) in the right regions.
Is there proof of availability of iron ore deposits in a concentrated form? A large amount of iron distributed thinly over the planet would not be very useful, unless it is dust and can be separated from dunes magnetically without excessive handling of non useful minerals.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#5 2020-02-11 19:47:32
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
Louis created this topic after finding a report of active research on use of iron as an energy storage medium.
Despite the 10 to 1 energy deficiency per unit of mass compared to coal, I remain interested in the potential use of iron as an environmentally benign energy storage medium.
However, iron powder is not without its risks.
I asked Mr. Google about risks of iron powder, and quickly learned that iron powder is hazardous to human health.
Thus, iron powder would need to be handled with precautions to avoid release, which would put it pretty much in the same category as oil, natural gas, ammonia or most other energy storage media.
Google: hazards of iron powder
People also ask
Is iron powder harmful?
Toxicological Effects: Chronic inhalation of finely divided iron powder may cause chronic iron poisoning and pathological deposition of iron in the body tissue. Ingestion may cause vomiting, diarrhea, pink urine, black stool, and liver damage.
www.espimetals.com › index.php › 917-msds › 935-iron-powderIron Powder - ESPI Metals
Perhaps iron powder could be packaged in a liquid of some kind which itself would serve as an energy carrier. I took a quick look at ammonia, and noted that iron and ammonia do not react until they are heated to (about 500 Centigrade). I'm not sure what form a mixture of these two materials would take. Ammonia can be liquefied at a relatively modest low temperature. On balance, ammonia hazards combined with iron powder hazards looks (to me at least) unappealing.
(th)
Last edited by tahanson43206 (2020-02-11 19:48:13)
Offline
Like button can go here
#6 2020-02-11 20:59:30
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,271
Re: Iron - the natural fuel for Mars?
Tahanson, how about Iron Pentacarbonyl? That is a room temperature liquid that can be pumped or sprayed into a boiler or rocket engine combustion chamber.
There is already a separate thread of CO as a fuel. Carbonyls may be easier to handle. Though they may not be an efficient way of storing energy.
https://en.m.wikipedia.org/wiki/Metal_carbonyl
Last edited by Calliban (2020-02-11 21:05:33)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#7 2020-02-12 10:19:32
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
For Calliban re #6
Thank you for what (at first impression) looks like a most interesting suggestion!
The issue of dangerous iron powder is immediately addressed. In addition, a property of the liquid is (according to Wikipedia) a pungent oder, which would be useful as a non-lethal warning to humans that there is a leak somewhere in the plumbing.
In addition, a power plant set up to release oxygen from rust produced by combustion of iron powder would be able to pull CO2 from the air to create CO for the reaction with iron, so the result would be carbon neutral, which is an acceptable substitute for carbon ** free ** given the lethal risks of going carbon free.
What remains to be determined is the energy carrying content of the liquid. From the table of relative properties you published earlier in this topic, I derived the understanding that iron has an energy carrying capability about 1/10th that of coal. I would be interested in knowing the energy carrying content of the liquid you have suggested. Here is a quote from Wiki via Google:
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)₅. Under standard conditions Fe(CO)₅ is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. Wikipedia
Molar mass: 195.9 g/mol
Formula: Fe(CO)5
Density: 1.45 g/cm³
Boiling point: 217.4°F (103°C)
ChemSpider ID: 24254
From Google we have:
DENSITY OF IRON AND IRON ALLOYS. The density of pure iron is 7.874 g/cm3 (491.5 lb/ft3, 0.284 lb/in3) at room temperature.
amesweb.info › Materials › Density_of_Iron
So now the rail cars for my imaginary train of iron powder are converted to tank cars, and they are larger than the hopper cars for the same mass of iron.
However, I surmise that the hopper cars are still needed for the return of rust powder to the power plant where the conversion would be performed. Perhaps the cars would be hybrid designs, able to contain liquid or powdered rust. A removable flat lid over a standard rectangular solid hopper car form might be a satisfactory solution, because the only concern (I can think of) is to keep the liquid from sloshing out of the car, and for keeping stray objects from falling into the container of liquid.
This system for capturing solar or wind energy is competing (conceptually speaking of course) with manufacture of methane or ammonia at a solar or wind plant.
There are too many trade-offs for this short post, even if I could think of all of them, which is unlikely.
Hopefully someone in the forum readership will be inspired by this discussion to toss in a guiding thought or two.
The goal is to come up with an energy capture and storage system that would be competitive on Earth, and useful on Mars.
(th)
Last edited by tahanson43206 (2020-02-12 10:24:37)
Offline
Like button can go here
#8 2020-02-12 11:42:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
Iron powdering has also a second benefit as the energy needed to process has already been expended to get it to a storeable form in that it is no longer oxide as in rusty. Its that powdered form that can allow us to use it in other manners. As in metal in expoxies for metal hole filling. Finishing it into a 3d processed object ect...
Offline
Like button can go here
#9 2020-02-12 12:10:17
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
For SpaceNut re #8
Thanks you for your addition to the topic.
As you noted in the preceding post(s), iron powder is harmful to human health. That is why I asked for assistance, and why Calliban suggested a liquid composed of iron particles and a carbon monoxide binder. The resulting liquid can be transported safely (I gather) and while it has an obnoxious odor, that odor is (apparently) not harmful to humans.
What I am hoping other forum members may find time to add is information about how well the proposed liquid energy carrier would function in a real world application, such as replacing entirely the fossil fuel for the many fossil fuel power plants around the world.
As In understand the original research which Louis cited in launching the current set of topics about iron, there exists a way to burn iron powder which can be adapted for a standard fossil fuel burning power plant.
(th)
Offline
Like button can go here
#10 2020-02-12 12:36:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
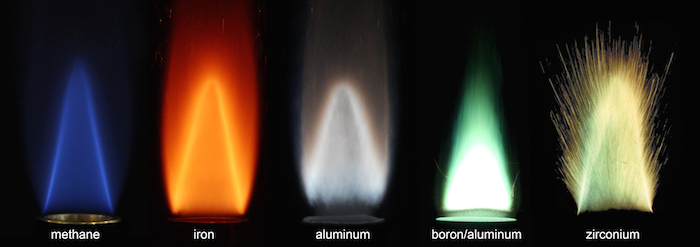
Notice the first flame is CH4 methane with O2 for the oxidizer to say that we are carbon nuetral in that the source of methane was from recycled co2 and H2 to manufacture it. Its combustion is however are not carbon nuetral.

Now using a metal powder and o2 for the oxidizer makes the metal obsorb the oxygen mostly when burned the color tells the heat content when burned. This is more about carbon nuetral exhaust rather than of energy storage or of efficiency of return.
To restore the metal that is now burned to an oxide means that we would smelt the metal with co to make it pure once more and the exhaust from that process needss to be capture so as to keep it nuetral.
One would cool the co2 and electolysis of it to break it back down into o2 and co for use once more.
Last edited by SpaceNut (2020-02-13 17:40:53)
Offline
Like button can go here
#11 2020-02-12 14:44:41
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Iron - the natural fuel for Mars?
Not necessarily.There is a European team working on coupling intermittent green energy with iron as a fuel, which is then effectively used as storage. The iron residue can be recycled into iron oxide, to be used again.
https://mcgill.ca/newsroom/files/newsro … orson1.jpg
Notice the first flame is CH4 methane with O2 for the oxidizer to say that we are carbon nuetral in that the source of methane was from recycled co2 and H2 to manufacture it. Its combustion is however are not carbon nuetral.
https://mcgill.ca/newsroom/files/newsro … _small.png
Now using a metal powder and co2 for the oxidizer makes the metal obsorb the carbon mostly when burned the color tells the heat content when burned. This is more about carbon nuetral exhaust rather than of energy storage or of efficiency of return.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#12 2020-02-12 19:12:10
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
Red iron(III) oxide (Fe2O3, commonly known as rust) is the most common iron oxide but there are others.

Iron reacts with simple substances: oxygen, halogens (bromine, iodine, fluorine and chlorine), phosphorus and sulfur. When iron is burnt, metal oxides form. Depending on the conditions of the reaction and the proportions between the two participants, iron oxides can differ. The equations of the reactions:
2Fe + O₂ = 2FeO; Iron (II) oxide FeO forms in the breakdown of ferric oxalate in an inert atmosphere, and is a black powder.
4Fe + 3O₂ = 2Fe₂O₃;
3Fe + 2O₂ = Fe₃O₄.
These reactions take place at high temperatures.
Thermite is a mix of iron and aluminum powder that when heated enough will start reacting with oxygen and burning at a high temperature — enough to melt metal and weld. The melting temperature of iron is 1538 degrees Celsius, and the boiling temperature is 2862 degrees.
When iron is burned in oxygen or in air, iron cinder forms, the equation reaction is:
3Fe + 2O₂ = Fe₃O₄, or
3Fe + 2O₂ = FeO • Fe₂O₃.
To obtain the oxide, a little fine iron powder is mixed in a ceramic pot with sodium nitrate or potassium nitrate. The mixture is heated over a gas burner. When potassium and sodium nitrate are heated, they break down, with the release of oxygen. Iron burns in oxygen, forming the oxide Fe₃O₄. After combustion ends, the obtained oxide remains on the bottom of the ceramic pot in the form of iron cinder.
Offline
Like button can go here
#13 2020-02-13 08:02:27
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
For SpaceNut re #12
Thanks for the helpful addition to the thread!
It is not clear (to me at least) what process the company in the Netherlands is using to process iron powder to deliver controlled heat for industrial use, but the end product must be "iron cinder" as your post describes.
It is that cinder the Netherlands company is apparently recovering in some efficient way so that it can be returned to a green power station to be restored to pure iron powder.
The contribution made by ** this ** discussion is the proposal of Calliban to combine the recovered iron powder with CO to produce a liquid that is non-toxic to humans for transport back to the power station.
The density of the proposed liquid is less than pure iron, but (at this point) I don't know how the density of the liquid compares to the density of iron powder. I would imagine that the density of iron powder would vary over some range, depending upon the amount of air in the mixture.
In any case, the liquid would appear to be a superior mode for delivery of iron from refurbishing facility to power plant.
What remains "to be determined" is the energy content of the liquid. It may be higher than the energy content of iron powder.
The proposed transport liquid does have the disadvantage of containing Carbon, but that disadvantage can be compensated for by making CO from CO2 collected from the atmosphere at the refurbishing facility. In that case, the resulting energy delivery product would be carbon neutral.
I note that the current CEO of BP (In England) is proposing to convert the company from fossil fuels to all non-carbon fuels by 2050. The CEO is apparently proposing to achieve this while sustaining the value of the stock and earnings for shareholders. That will be quite a feat of management, and if accomplished, it will justify whatever high salary this individual is earning.
The process we are discussing in this thread would (or could) contribute to achievement of the goal, for BP, or for any company with a similar inclination.
(th)
Offline
Like button can go here
#14 2020-02-13 09:05:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
For SpaceNut re #10
First, thank you for the diagram which shows a form of the combustor design that swirls iron powder to allow it to fall into the collector bucket. I recall that as one of the features of the system designed by the group in the Netherlands.
However, I would like to offer an opportunity for a possible editorial change to the text you added at the bottom of #10. As the diagram shows, the metal powder is reacted with Oxygen, NOT with CO2. The intake of air is shown on the left of the diagram, and exhaust of Nitrogen is shown on the right. Presumably the oxygen has been consumed in combustion of the metal powder.
(th)
Offline
Like button can go here
#15 2020-02-13 17:45:03
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
Corrected content to reflect oxidizer and recycling of materials process.
The exhaust could have some co, co2, no2 impurity of so2 and others.
I think the swirling is to cool the powder such that it can not form a droplet to make it easier to reduce later back to a pure form.
Offline
Like button can go here
#16 2020-02-13 19:21:09
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,502
Re: Iron - the natural fuel for Mars?
For SpaceNut re #15 and correction to #10
Thanks for the adjustment.
Your list of Sulfur Dioxide as a pollutant in the exhaust from burning iron is a surprise, because incoming air should contain little sulfur.
The table at the link below shows Sulfur Dioxide as a trace constituent of "normal" air.
https://eesc.columbia.edu/courses/ees/s … ble_1.html
Sulfur Dioxide ** is ** a contaminant from burning coal, but the process under discussion here is burning iron, not coal.
That said, I agree that if the iron powder is carried in a liquid mixture (called Iron Pentacarbonal) then the CO present in the mixture might not burn completely with oxygen, so some CO could be expected in the output.
Oxides of Nitrogen would be an unwanted byproduct of combustion of just about anything with air.
Mr. Google found this:
NOx is produced from the reaction of nitrogen and oxygen gases in the air during combustion, especially at high temperatures. In areas of high motor vehicle traffic, such as in large cities, the amount of nitrogen oxides emitted into the atmosphere as air pollution can be significant.
It turns out that a number of people have asked Google about the greenhouse gas properties of nitrous oxide:
Nitrous oxide has an atmospheric lifetime of 110 years. The process that removes nitrous oxide from the atmosphere also depletes ozone. So nitrous oxide is not only a greenhouse gas, but also an ozone destroyer. Nitrous oxide (or N2O), is more commonly known as laughing gas.Dec 8, 2014
This observation may have a chilling effect on the attraction of burning iron as an alternative to coal on Earth.
However, it would clearly NOT be a concern on Mars, but in the case of Mars, the non-availability of Oxygen would be a challenge.
Ths line of discussion has proven less fruitful than I had hoped. Iron might help with reduction of Carbon Dioxide, but it wouldn't reduce production of Nitrous Oxide at all.
Thanks to everyone who contributed!
(th)
Last edited by tahanson43206 (2020-02-13 19:23:21)
Offline
Like button can go here
#17 2020-02-13 20:41:39
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
Since we need a pure source for oxygen and we can draw off the nitrogen and make use of water electrolysis on just wants to combine a few things to make a better inlet of air for the process to work better.
We know how to filter and cyrogenic cool the nitrogen and oxygen out of the air. We also know how to purify water of contaniments for use in making hydrogen to combine with the nitrogen fro the other topic. The remaining super cooled oxygen when add to the oxygen from the electrolysis makes for a very clean source of oxygen for the process.
These things fix the outlet exhaust from burning to very low percentage of anything but unburnt oxygen.
Offline
Like button can go here
#18 2023-05-12 13:16:42
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Iron - the natural fuel for Mars?
In an Incredible First, Scientists Have Discovered What's at The Core of Mars
https://www.sciencealert.com/in-an-incr … re-of-mars
Local space colony Resource Utilization
The wonderful products that can be created with power and the dangerous mix of chemistry inside the Biosphere and Manufacture Tunnels of Mars.
Very high temperature and fine powder mixture of metal oxide and a metal that will act as a reducing agent? Making Ferroniobium from niobium pentoxide and ferrovanadium from iron, vanadium(V) oxide, and aluminium, a process begins with the reduction of the oxide by the aluminium, the Inorganic reaction and Metallurgy topic
3 V2O5 + 10 Al → 5 Al2O3 + 6 V
and Fe and AL chemical reaction
Fe2O3 + 2 Al → 2 Fe + Al2O3
Aluminothermic reactions are exothermic chemical reactions using aluminium as the reducing agent at high temperature. The process is industrially useful for production of alloys of iron, a prominent example is the thermite reaction between iron oxides and aluminium to produce iron itself.
some other Heat topics
Iron and Steel on Mars
https://newmars.com/forums/viewtopic.php?id=6133
Power generation on Mars
https://newmars.com/forums/viewtopic.php?id=220
Thermal heat storage
https://newmars.com/forums/viewtopic.php?id=9229
Kitchen Design / Appliances / Practices for Mars
https://newmars.com/forums/viewtopic.php?id=10411
Mars’ emitted energy and seasonal energy imbalance
https://www.pnas.org/doi/10.1073/pnas.2121084119
Thermite reactions with oxides of iron and silicon during combustion of magnesium with lunar and Martian regolith simulants
https://www.sciencedirect.com/science/a … 8015001789
Magnesium and carbon dioxide - A rocket propellant for Mars missions
https://arc.aiaa.org/doi/10.2514/3.23609
Aluminothermic reactions have been used for welding rail tracks, Mars has Dust storms and electrical discharge one use is the welding of copper cables wire for use in direct burial (grounding/earthing) applications.
Mars Rail discussion
https://newmars.com/forums/viewtopic.php?id=3501
'Trains on Mars - Could a rail system provide martian need'
Offline
Like button can go here
#19 2023-06-01 05:38:52
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Iron - the natural fuel for Mars?
ESA is Testing How Iron Burns in Weightlessness
Offline
Like button can go here
#20 2024-06-12 18:00:38
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,932
Re: Iron - the natural fuel for Mars?
Seems we can even use the thermite reaction on the moon as well.
Using the moon's soil to support life, energy generation and construction
A research team from the University of Waterloo's Laboratory for Emerging Energy Research (LEER) is looking into processing lunar regolith, the moon's top layer of soil and dust, into usable materials for life support, energy generation and construction. This includes investigating the use of defunct satellite material as a fuel source when mixed with lunar regolith. The International Astronautical Federation has published two papers on the research.
Offline
Like button can go here