New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2024-02-14 09:53:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Heat Pump - Heat Pumps
For SpaceNut ... It appears we did not have a topic dedicated to heat pumps, but they have appeared many times in this forum, under a variety of topics.
This topic is available if members would like to collect links and text, and perhaps even images about the technology.
My interest is primarily in where the sweet spot exists in particular situations.
It takes energy to operate a heat pump.
When there is sufficient spread between the high temperature and the low temperature in the system, then (I gather) the return on that energy investment is greater than the investment in the pumping.
(th)
Offline
Like button can go here
#2 2024-02-14 09:54:29
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
This post is available for an index to posts that may be added to the topic over time.
Below is a link to a conversation with Gemini about online COP calculators and other calculators for Heat Pumps
https://docs.google.com/document/d/1_m9 … sp=sharing
Update: Calliban uses the term COP in the posts below...
Coefficient of performance
Wikipedia
https://en.wikipedia.org › wiki › Coefficient_of_perfo...
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling ...
and in more detail:
From Wikipedia, the free encyclopedia
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required.[1][2] Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP is used in thermodynamics.
The COP usually exceeds 1, especially in heat pumps, because instead of just converting work to heat (which, if 100% efficient, would be a COP of 1), it pumps additional heat from a heat source to where the heat is required. Most air conditioners have a COP of 2.3 to 3.5. Less work is required to move heat than for conversion into heat, and because of this, heat pumps, air conditioners and refrigeration systems can have a coefficient of performance greater than one.
The COP is highly dependent on operating conditions, especially absolute temperature and relative temperature between sink and system, and is often graphed or averaged against expected conditions.[3]
Performance of absorption refrigerator chillers is typically much lower, as they are not heat pumps relying on compression, but instead rely on chemical reactions driven by heat.[4]
Equation
(th)
Offline
Like button can go here
#3 2024-02-14 11:07:56
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
On Mars, there are plenty of locations where daytime soil temperature exceeds 0°C.
https://en.m.wikipedia.org/wiki/Climate … emperature
If we can gather that heat using flat plate collectors, it could be stored by melting ice contained in tanks. The latent heat of melting of ice is 334KJ/kg.K. That means that 1m3 of water will store some 93kWh of heat through phase change. The tank could contain a heat exchanger coil for a heat pump. A heat pump supplying heat at 30°C from a cold source at 0°C, will have a theoretical COP of 9.1. The lower the temperature rise the better the COP. If the pump supplies heat at a temperature of 20°C, the theoretical COP will be 13.65. When we build structures on Mars, we should embed plastic water pipes within the floors and walls. By doing that, all surfaces can be turned into heat transfer surfaces and the water we use to heat spaces can be supplied at close to the desired air temperature. Than way, we get the most heating value out of each unit of power supplied to the heat pump.
Heat pumps could be used at higher and lower lattitudes where temperatures are lower. But COP declines rapidly. If daytime temperatures are -20°C and we are supplying heat at 20°C, the COP can be calculated accordingly:
COP = Tc/(Th-Tc) = 253/40 = 6.33
This tells us that a heat pump drawing heat from a -20°C cold side will need twice as much mechanical energy as a heat pump drawing from a 0°C source.
If the base is equipped with a nuclear power source producing waste heat at a temperature of 30°C, we might use this for heating without need for heat pumps at all. But to heat using water at such low temperature, heat transfer surfaces must be large. Which is why I think Martian buildings should have heating pipes in their walls and floors.
Last edited by Calliban (2024-02-14 11:16:43)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#4 2024-02-14 11:38:00
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
On Mars, fine regolith has about the same thermal conductivity as rockwool on Earth. This makes it a very good thermal insulator. Our flat plate solar collector could be an area of flat ground that has been excavated to a depth of 6" and then filled with sieved regolith. A plastic hose pipe would be coiled in rows on top of the regolith and then covered with a thin layer of flat rocks. These will absorb heat and conduct it into the pipe. The pipe would contain methanol. This has a low vapour pressure at 0-20°C, and a low freezing point of -97°C.
https://en.m.wikipedia.org/wiki/Methanol
This means that close to the equator, even the cold Martian night will not be sufficient to freeze it. During the day, the methanol temperature will rise above freezing. Natural convection will cause it to rise into coils within the ice tank. This allows the ice tank to gather heat by natural convection, without any moving parts. The ice tank can be made from compacted soil or adobe bricks, with a polymer membrane to contain the liquid. The bottom of the tank will be cone shaped. As water freezes and ice expands, the ice is pushed upward, rather than exerting pressure on the walls of the tank.
Last edited by Calliban (2024-02-14 11:42:32)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#5 2024-02-14 12:19:54
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
Top down view: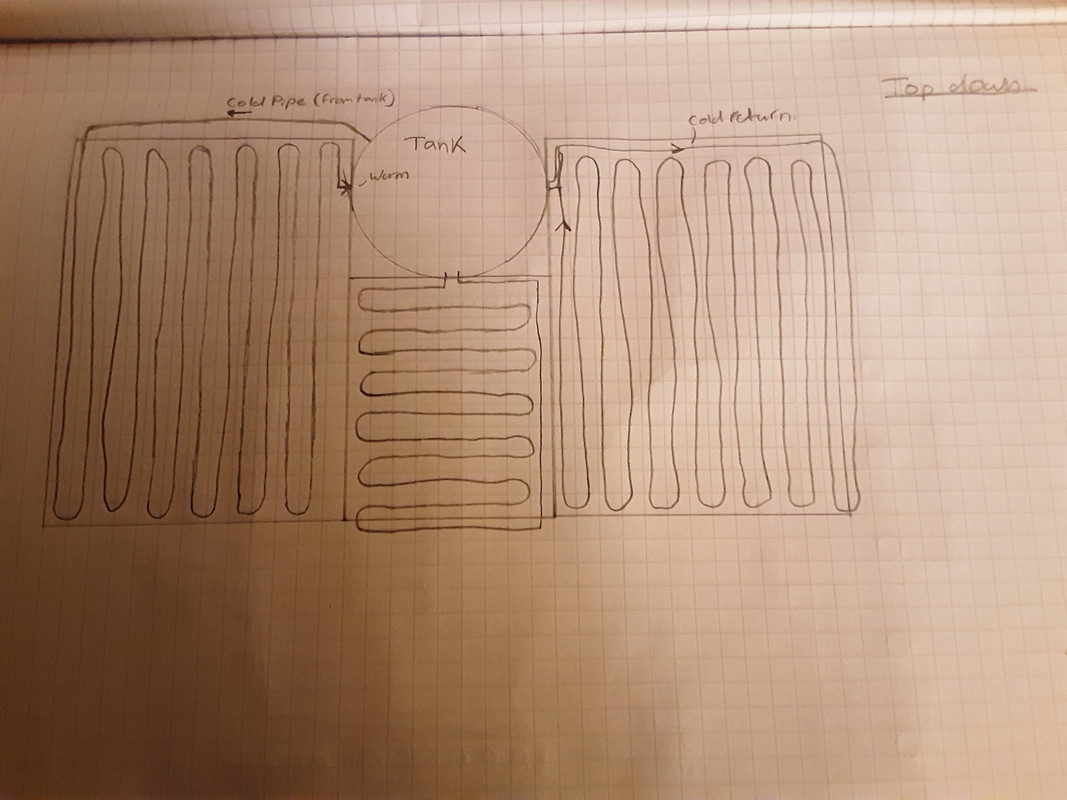
Cross section of collector: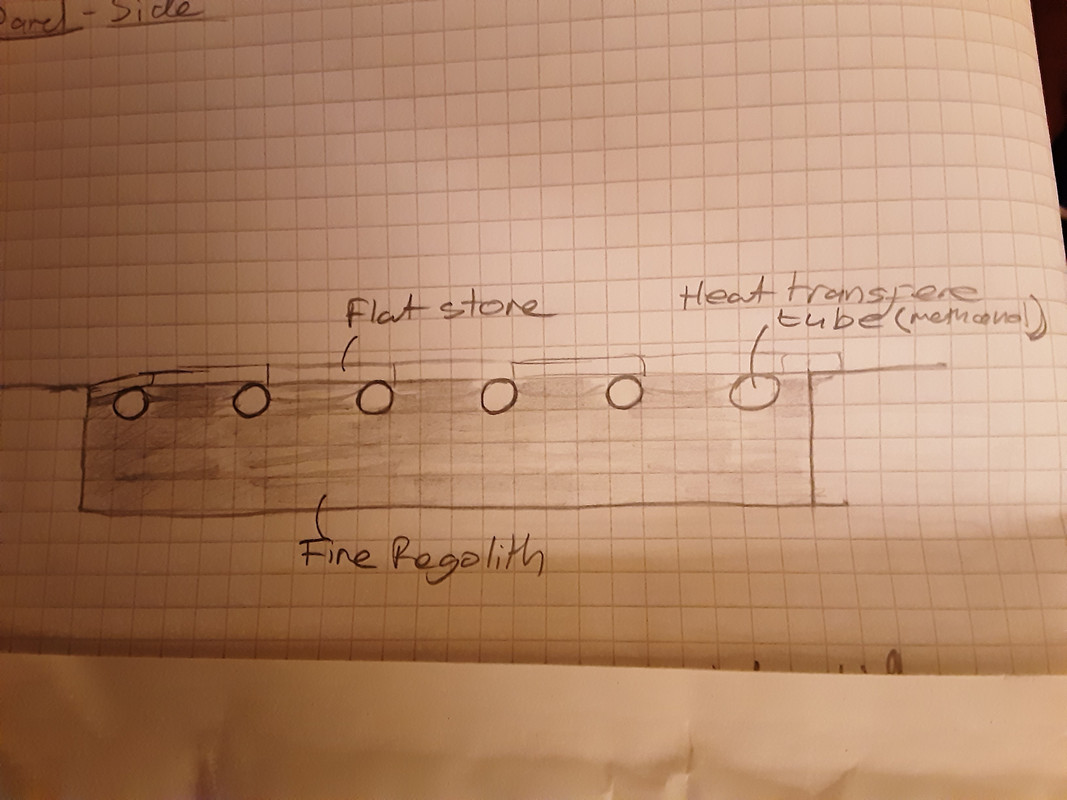
The heat storage tank:
Warm methanol flows up the header pipe to the top of the tank and then descends through the coiled pipe embedded within the tank walls, teansfering heat into the tank melting the ice. Cold methanol then returns to the collector panels. The heat pump cold side heat exchanger is at the bottom of the tank. This withdrawns heat at 0°C.
Last edited by Calliban (2024-02-14 12:27:26)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#6 2024-02-14 17:50:58
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
You also can use evacuated-tube collectors.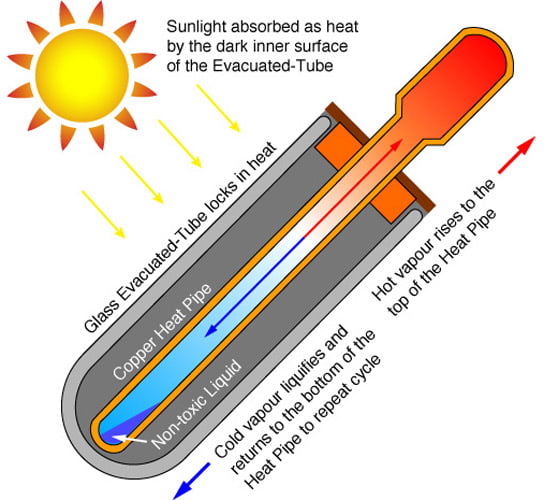
for cold areas you design a mixture of 30% propylene glycol and 70% water is often used as insurance against catastrophic system failure.
Drainback Solar Thermal System Design
Offline
Like button can go here
#7 2024-02-15 08:22:19
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
For SpaceNut re #6
Thank you for this interesting report on what looks like an effective way of collecting solar energy for heating.
(th)
Offline
Like button can go here
#8 2024-02-15 08:24:43
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
In this post, I will attempt to show a conversation with BARD about this topic as it related to a community in Alaska.
BARD did not provide a table of values that I was hoping for. There may be too many variables for such a table to exist.
However, BARD ** did ** find a location (Finland) where the ocean is a source of thermal energy.
Heat Pump Sea Water Alaska
Hello Again! This morning I have a question that may call upon your search skills, as well as your ability to organize complex ideas for presentation. Our group has a member who offers for consideration the idea of using a heat pump in association with a large body of water such as an ocean. He offers a suggestion that sea water might be drawn into a heat pump from under a layer of ice, processed so that it's thermal energy is given up to some degree, and then returned to the top of the layer of ice, so that the water under the ice is not cooled by the returned fluid. I understand that the energy required to operate the pump is less than if that energy had been used to heat a room directly. However, I feel sure that there must be a point at which the energy invested in pumping is greater than the thermal energy return. With your search skills, you may be able to find reports of analysis of these relationships, so that we might be able to advise a community located in Alaska as to whether the heat pump idea makes sense.
expand_more
volume_upThank you for the ** very ** encouraging report that there is an installation that draws thermal energy from the ocean in Finland. This location is a good match with the Alaska community, which is located on the coast of the inland waterway system that exists there. What I am hoping you might be able to find is a mathematical relationship between the elements of the situation we have described. An ocean may be considered to be an inexhaustible source of thermal energy, but the amount of thermal energy that can be drawn from sea water that is under a layer of ice may be limited by practical considerations we are hoping you can help us to discover. A village along the coast of Alaska has two sources of energy right now. The local supply of wood can heat homes during the winter, and oil can be imported from elsewhere in Alaska to heat homes and businesses. What we are trying to learn is whether the fossil fuel imported from elsewhere in Alaska might be more effectively used to pump heat from the ocean, than to heat air in a furnace as is done at present. Do you have access to mathematical tables that show the effectiveness of heat pumps in various situations?
expand_more
volume_upThank you for laying out an overview of some of the factors that might be considered for this application. It seems to me that the experience of the folks in Finland might be most helpful. I'm hoping they kept records of all their investments, and the results achieved, and might be willing to share their experience with others.
edit
Show drafts
Here is a link to the transcript.
https://docs.google.com/document/d/1GBS … sp=sharing
The transcript includes information about the Finland project.
(th)
Offline
Like button can go here
#9 2024-02-15 08:45:01
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
My impression is that BARD took a larger view of my request than was needed.
The art of phrasing a request is clearly one I have not yet mastered.
All I wanted was a graph showing the trade point where the energy invested in pumping exceeds the energy delivered.
The term COP was introduced by Calliban into this topic, and I added an explanation of the term in Post #1.
I gather that a COP of 1 is an indication the heat pump system is not worth while.
BARD seemed worried about the depth of the ocean. I see no reason whatsoever why that is relevant. All that's needed (as far as I can see) is a source of liquid water that is under the ice. Any water drawn from under the ice will be replenished by the rest of the ocean.
I followed up with BARD, and that conversation is appended to the transcript linked above.
In this phase of the conversation, the thermal collection concept focused on the phase change energy of water to ice.
BARD gave the energy for this change, and that is included in the transcript.
What I am curious to find out is whether the energy invested to remove thermal energy from water that is just below freezing is great or less than the thermal energy recovered. I think the correct term might be COP ... Coefficient Or Performance..
If the COP is 1 or less, then the idea of harvesting thermal energy from the phase change is not useful.
Update: it appears that the concentration of salt in sea water is (on average) 35 grams per liter, so the freezing point is -1.9 degrees Celsius.
Factors to consider:
Heat of fusion of water: This is the amount of energy required to melt 1 kg of ice (334 kJ/kg). This will be the primary source of thermal energy extracted in your system.
Specific heat capacity of water: This is the amount of energy required to raise the temperature of 1 kg of water by 1°C (4.18 kJ/kg°C). This will be relevant if you intend to extract additional heat after the phase change.
Temperature difference: The greater the difference between the initial water temperature and the desired output temperature, the more thermal energy you can extract. However, remember that extracting excessive heat might lead to solidification within the system itself.
Flow rate: The amount of water processed per unit time will determine the total energy output.
Efficiency of the heat pump: While not directly applicable to phase change, if other heat transfer processes are involved, the pump's efficiency will impact the overall energy output.
I presume that there must be 334 kJ/kg of thermal energy to be harvested from the water under the ice, if the water can be processed so that it does not freeze in the equipment. How ** that ** might be achieved is an exercise for the reader.
And! It seems reasonable (to me at least) to assume that the energy required to harvest that thermal energy must be less than 334 kJ/kg.
(th)
Offline
Like button can go here
#10 2024-02-15 09:10:18
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
TH, COP stands for 'coefficient of performance'. It is a ratio of the thermal energy we get out of a heat pump, divided by the mechanical energy needed to drive the compressor. COP needs to be substantially above 1 for the heat pump to be worthwhile. A COP of 1 is what you get from a heating element. The higher the COP, the better. The simple COP values I calculate here are carnot efficiencies for reverse heat engines. Real COP will be anywhere between one half and two thirds of this value.
Larger heat pumps can be expected to have better COP, as larger machines have fewer 'irreversible' effects, like friction, thermal losses, etc. The same is true for larger engines and powerplants. Very large powerplants can achieve up to 75% of carnot efficiency (i.e boiling water reactors). But that requires GW scale plants. For reverse heat engines, the same scaling effects apply. But the problem is that heat cannot be transmitted long distances without substantial losses. So the heat pump must be relatively close to the heat load. That drives down the optimum size of reverse heat engines.
Last edited by Calliban (2024-02-15 09:16:04)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#11 2024-02-15 09:50:18
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
For Calliban re #10
Thank you for the review of COP, and for examples of machines with varying efficiency.
I was unable to determine if sea water under a layer of ice can be harvested. The water directly under the ice would appear to be at -1.9 Celsius, but the water further down would presumably be warmer, since the ocean is continuous throughout the planet. The scenario I attempted to investigate is harvesting thermal energy from sea water that is at -1.9 Celsius. I ** think ** there should be thermal energy to be harvested if the water can be kept from freezing during harvesting. The output would be delivered to the top of the ice, where it would remain until the spring thaw.
What I'm unclear on is whether the energy needed to perform the harvest is greater or less than the energy delivered.
Update ... what I appear to be talking about is an ice making machine. Ice making machinery has been in global use for many many years. The waste heat produced is (most likely) considered a nuisance that must be discarded.
In the scenario I'm considering for the Alaskan village, the fossil fuel that might be used to deliver heat using hot air furnaces would instead be used (somehow) to drive an ice making machine. The waste heat from ** that ** operation would be used to heat homes.
The question I am asking is... if the ocean is the source of the water to be turned into ice, is the COP greater than 1?
(th)
Offline
Like button can go here
#12 2024-02-15 12:02:56
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
After spending some quality time with BARD, I decided to take the Ice Machine question to ChatGPT4. It concluded that a COP of 4 is within the realm of possibility, for the ice machine scenario. I'll paste the report CH4 generated, and then an image that DALL-E came up with.
Summary of Discussion on Thermal Energy Extraction Using Ice Machines in Alaska
Our conversation explored the feasibility and environmental implications of utilizing sea water to extract thermal energy, with the by-product being ice. This idea stems from discussions on heat pumps and seeks to leverage the natural cold temperatures and abundant sea water in Alaska for sustainable heating solutions.
Key Points Discussed:
Concept Overview: The idea involves drawing sea water, extracting thermal energy (thus cooling the water), and then returning the material in the form of ice back to the environment. This process aims to reduce reliance on fossil fuels for heating, leveraging the principle of heat pumps.
Coefficient of Performance (COP): We discussed the importance of achieving a COP greater than one, which would indicate efficiency gains compared to direct electrical heating. A hypothetical COP of 4 was used for illustrative purposes, suggesting that the system could deliver heat at a rate four times what could be expected from the electricity alone.
Environmental Considerations: Key environmental considerations include the corrosion and material compatibility due to sea water's corrosive nature, the freezing point depression caused by salt, and the potential environmental impact of reintroducing brine and ice into the ocean.
Scale and Impact: We acknowledged that the scale of human activity, especially in a localized context such as a village, is unlikely to significantly impact the vastness of the ocean. The process mimics natural sea water freezing, where salt is excluded from the ice, suggesting minimal ecological disruption.
Revised Scenario: Adjusting the scenario to a location where the ocean surface never freezes over, we concluded that ice cubes and brine reintroduced into the ocean would rapidly return to equilibrium with minimal environmental impact. This reinforces the sustainability of the proposed method.
Conclusion: The innovative approach to using sea water for thermal energy extraction in Alaska presents a promising pathway for sustainable and efficient heating. By leveraging natural processes and carefully considering environmental impacts, such systems could offer significant benefits over traditional fossil fuel-based heating methods.
This summary is designed for a small Internet forum and formatted in BBCode for easy integration. The discussion encapsulates a blend of technical feasibility, environmental stewardship, and the practical considerations of implementing such a system in a challenging yet resource-rich environment like Alaska.
Link to image goes here: 
(th)
Offline
Like button can go here
#13 2024-02-15 18:59:01
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
The heat of day and cold of night means we are making a differential style AC / heat pump with solar from other sources to drive energy stored such that it can create power as well. This is the MIT design using auto parts create in Africa from the peace corp activity to bring power to the rural areas.
Offline
Like button can go here
#14 2024-02-16 07:30:17
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
It takes energy to extract thermal energy from a material.
COP is the abbreviation for the term that Calliban has pointed out as the measure of efficiency of the method.
A Coefficient of Performance greater than 1 is desirable for a heat pump system.
I would like to see this topic contain a description of how much energy is consumed extracting thermal energy from various materials in various situations.
A model scenario is at hand. We can pull sea water from an ocean at a temperature of -1.9 Celsius, and we can extract thermal energy until the sample is near to absolute zero.
The total amount of thermal energy available in that sample from the ocean should be knowable.
The amount of energy needed to extract that thermal energy should be knowable.
A chart showing the performance of such a system should be possible.
The COP may vary as the temperature of the sample decreases.
There may be a range over which the procedure is productive.
It may be possible to design a computer program that can deliver a performance chart for any material over any desired temperature range.
(th)
Offline
Like button can go here
#15 2024-02-16 09:01:28
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
Quoting from Wikipedia:
From Wikipedia, the free encyclopedia
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required.[1][2] Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP is used in thermodynamics.
COP was brought to our attention by Calliban.
A Heat Pump can be understood as a force multiplier.
Energy is invested to move thermal energy from where it is available to where it is needed.
A COP of 4 means that 1 unit of energy invested yields 4 units of energy in the desired location.
In this topic, we are looking at a possible opportunity in Alaska.
Much closer to most of our members, we have water in the Earth's mantle.
That water is warmed by energy from the Earth's core.
It should be possible to harvest thermal energy from water under the surface.
With any luck at all, this topic may/should/could become a collection point for knowledge of how to do that, and more importantly, how effective a heat pump would be for this purpose, under various circumstances.
Update:
Below is a link to a conversation with Gemini about online COP calculators and other calculators for Heat Pumps
https://docs.google.com/document/d/1_m9 … sp=sharing
Per the transcript above, here is the Engineering Toolbox Heat Pump Calculator
The example for a home shows -5 Celsius outside, and 40 Celsius inside.
Alaska scenario is above that low end, at -1.9 Celsius.
(th)
Offline
Like button can go here
#16 2024-02-16 11:17:53
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,646
Re: Heat Pump - Heat Pumps
I asked ChatGPT4 to consider the question of using an ice making machine as the foundation for a heat pump to work in the Arctic environment, with water at -1.9 Celsius as input and ice as the waste product, while heat becomes the valuable output. As may occur to the reader, this is the inverse of purpose for which the ice machine was invented in the 1850's.
Summary of Discussion on Innovating Ice Machine Technology for Thermal Energy Extraction
This discussion explored the potential for adapting ice-making technology to extract thermal energy from sea water, focusing on sustainability, technical feasibility, and identifying potential industry partners for collaboration.
Key Discussion Points:
Innovative Concept Overview: We discussed reversing the traditional ice machine's operation, aiming to produce heat as the primary output and ice as a by-product. This involves utilizing the thermal energy extracted during the refrigeration cycle for heating purposes, particularly in cold climates like Alaska.
Principles of Heat Pump Operation: Reviewed the fundamental operation of heat pumps, emphasizing the importance of refrigerant selection and system design to ensure efficiency in extracting thermal energy from cold environments.
Environmental Considerations: Highlighted that, when scaled appropriately, this technology could offer a sustainable heating solution with minimal environmental impact, similar to natural processes.
Potential Industry Partners for Collaboration:
Carrier Corporation: Recognized for HVAC and refrigeration innovation, Carrier's extensive R&D might support adapting ice-making systems for energy extraction.
Daikin Industries: As a global leader in air conditioning and refrigeration, Daikin is well-positioned to explore novel applications of refrigeration technology.
Trane Technologies: With a focus on sustainability and HVAC systems, Trane could be interested in the project's environmental benefits.
Emerson Electric Co.: Known for engineering excellence in refrigeration, Emerson could provide the technical expertise needed to bring this concept to fruition.
Next Steps:
- Research and Outreach: Conduct in-depth research on these companies, focusing on their commitment to innovation and sustainability. Prepare a compelling proposal that outlines the concept and its benefits.
- Engage with Potential Partners: Use industry events and direct outreach to initiate discussions, emphasizing the mutual benefits of developing this technology.Conclusion:
This innovative approach to using ice-making technology for thermal energy extraction presents a promising opportunity for sustainable heating solutions. Collaboration with industry leaders known for their technical prowess and commitment to innovation could accelerate the development and implementation of this technology.
For further discussion or collaboration, feel free to reach out or continue the conversation in this forum.
It would appear that contact with the major players listed in the report is the logical next step.
(th)
Offline
Like button can go here
#17 2024-02-16 17:10:58
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
Simply the 4 ton heat pump needs 4kw to power to generate 48,000 BTU per hour. Convert BTU To Watt: Calculator + Conversion Chart (1 BTU = 0.293 W). is 14,068 watts of heat energy coming out of the unit to spread across the home. 1000W heat pump with a COP of 3.5. It will boil almost 10 gallons of water per hour.
COP = Q/W
where Q is the heat, the heater generates if we give it a certain amount of work (W). or COP heat pump = T hot/(T hot-T cold)
Offline
Like button can go here
#18 2024-02-17 16:52:29
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
Offline
Like button can go here
#19 2024-02-19 18:05:08
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
The Many Advantages of a Closed-Loop Geothermal System
Geothermal heating and cooling is a great way to lean into sustainable energy, and a long-term cost-effective solution that'll benefit your home and family and increase property value. Whether you adopt a vertical system or a horizontal configuration, geothermal energy systems harness the Earth's natural heat to promote energy savings and reduce your carbon footprint.
"Closed loop geothermal (CLG) systems are geothermal systems that don’t exchange water with the external environment, but instead run water through a 'closed loop,' or a 'closed circuit,'" explains Kathy Hannun, Founder & President, Dandelion Energy. "The system’s underground pipes are charged with water during installation, and then sealed so that the same water circulates through those underground loops indefinitely."
Open loop systems refer to another type of geothermal system where water is pumped out of the ground, run, through the geothermal heat pump, and then dumped back into a nearby body of water (like a pond) or approved drainage ditch.
Advantages of a Closed Loop Geothermal System
Fewer maintenance requirements. They require minimal maintenance because there are fewer components exposed to external elements.
Reduced levels of debris and risk of contamination. The systems are lower maintenance than open loop systems because groundwater can contain debris or mineral content that can clog or contaminate the indoor heat pump.
Less affected by fluctuations in the groundwater levels. The systems simply aren't as vulnerable to changes in groundwater levels or flows.
Flexibility in installation. These systems can be installed in a variety of locations, including areas where access to groundwater is limited or the groundwater source is poor. This flexibility can make them a more practical option for a wider range of applications.
Higher efficiency ratings. Closed loop systems are often more efficient because they are less susceptible to fluctuations in groundwater temperature and other external factors. This can result in lower energy consumption and higher overall performance.
Longer lifespan. The piping system is typically buried underground, protecting it from exposure to the elements and minimizing wear and tear. As a result, closed-loop systems often have a longer lifespan than open-loop systems, providing more reliable heating and cooling over time.
Open loop then requires a recovery ond so as tpo not waste the water resources.
Offline
Like button can go here
#20 2024-02-20 08:02:44
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
The US is better placed than Europe to make use of ground source heat pumps at the household level, simply because houses have more space around them.
One common problem wherever you choose to build a heat pump - there is a conflict between COP and heating rate. Heating panels (i.e radiators) are more effective if there is a large temperature difference between them and the room they are heating. If a heat pump is recieving heat at 10°C and discharging heat to a panel which is at 30°C, the Carnot COP is 14. But the temperature difference between the panel and the room is only 10°C, assuming the room is at 20°C. Convective heat transfer into air is proportional to temperature difference. Now consider the case of a heat pump recieving heat at 10°C and discharging at 60°C. Temperature difference between the panel and room is now 40°C. So heating the house can be achieved with only 1/4 the area of heating panels. But Carnot COP has declined to 5.6, which is much poorer. Realistically, real COP will be around 3 under those conditions.
There are ways of mitigating these problems, but they aren't easy.
1. You can opt for lower temperature heating by increasing radiator panel area. But the panels would need to cover so much of your walls, that really you need to embed heating pipes within the walls and floors themselves. That means stripping the walls and floors and retrofitting the whole house. Expensive and bothersome.
2. You could stick with existing radiator panels and superinsulate the house, so that less heat flux is needed to maintain a comfortable temperature. People have tried that, but really the house needs to be built with that goal in mind for it to work. If you are stuck with an old victorian era terrace, then there is a limit to what you can do to insulate it.
3. You can tolerate a colder house and wear warmer clothes. That is the easiest option, but it isn't popular. People want to be warm. Women notice the cold more readily than men.
4. You can demolish the house and build a new one that is more optimised for heating in this way. Again, most people cannot afford. Replacing the house is going to cost more than most people spend on heating their entire lives.
5. You can take the financial hit and heat your house with a 60°C outlet heat pump. That works on a functional level, but if you live in a place like the UK, where electricity costs around $0.33/kWh, then it will cost more to heat your house in this way than it would if using gas. If you are struggling to pay the bills, why would you invest in something that is going to cost you more?
Right now, most people don't have heat pumps. In Europe where gas is expensive, the solution for most people is to tolerate the cold by investing in warmer clothing. People that are wealthy enough are burning gas and just paying the price. But heat pumps remain niche because people either cannot afford to buy them or don't have the space, or are wealthy enough not to care about the gas price. So that stick with a gas boiler and just take the financial hit.
Last edited by Calliban (2024-02-20 08:15:06)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#21 2024-02-20 10:48:10
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Heat Pump - Heat Pumps
Calliban,
One of the advantages of Victorian terraces is the high ceilings. You can afford to sacrifice a few centimetres of height to lay down underfloor heating panels. Screedless systems exist, so retrofitting is a possibility.
The trouble with costs for such things is, there's no standard pricing. Possibly because the people doing the installation have room to charge what they like.
Use what is abundant and build to last
Offline
Like button can go here
#22 2024-02-20 14:27:41
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,278
Re: Heat Pump - Heat Pumps
Calliban,
One of the advantages of Victorian terraces is the high ceilings. You can afford to sacrifice a few centimetres of height to lay down underfloor heating panels. Screedless systems exist, so retrofitting is a possibility.
The trouble with costs for such things is, there's no standard pricing. Possibly because the people doing the installation have room to charge what they like.
A heated ceiling with a recirculating fan is an interesting possibility, especially if the ceiling is comfortably above head height. Ceilings are plasterboard, which can be easily removed and replaced with a flat panel screwed or bolted to the floorboards above. This is something that wouldn't be too disruptive to install. The panel needs to be non- combustible. Maybe aluminium with the appropriate surface coating? The room I am sitting in has dimensions 4m x 8m. The single wall radiator is 1.5m2 in area. So ceiling area is over 20 times greater than my existing radiator area. My boiler is set to heat my radiators at 50°C, which is 30°C greater than room temperature. Using a ceiling heating panel, I would get the same heat flux into the room with a 20x smaller temperature difference, or 1.5°C. So to maintain room temperature at 20°C, radiator water temperature must be 21.5°C. Now we're getting somewhere.
Assuming my heat pump can access heat at 10°C, the ideal COP would be 24.6. Even if we can can only achieve half of that performance in a real system, with electricity costing £0.3/kWh, the cost of pumped heat would be £0.0244/kWh. That is about half the price of gas. A temperature of 21.5°C, is obviously too cold for wash water. But the heat pump could preheat the water going into an electric water heater, reducing the power needed.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#23 2024-02-24 11:36:17
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Heat Pump - Heat Pumps
I'm not a great enthusiast for air source heat pumps, but... With an appropriate low temperature heating system, we would be able to significantly reduce our gas consumption, even allowing for the use of gas to provide the electricity. Space heating is 3/4 of our energy use, and when it's 0c outside the theoretical CoP would be perhaps 12. 8 should be achievable in a real system. A quarter the gas needed for heating compared to a boiler. Keep the boiler for now to provide hot water, we shouldn't try to make the same heat pump do double duty for 60c hot water as for heating.
I'm thinking it could act as a bridge to a district heating system? If homes are already retrofitted with required heating systems, connecting up to the local heat grid when it comes requires far less work and disruption -- you're just replacing the air source with the heat grid source.
Looking at the costs for radiant ceilings though, and I'm pretty sure the prices are inflated over what they should cost, probably due to the paucity of installers. But screwing some panels to the joists shouldn't cost £100 per sq. m...
Use what is abundant and build to last
Offline
Like button can go here
#24 2024-03-16 08:44:47
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
Offline
Like button can go here
#25 2024-04-29 20:30:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,038
Re: Heat Pump - Heat Pumps
Was surprised to see how great the difference is for a furnace versus a heatpump
Most homeowners choose a 3-ton-capacity heat pump, which is good for about a 2,000-square-foot home, according to HomeAdvisor, a home-improvement services marketplace.
According to HomeAdvisor, the average cost to install one unit is $3,000, but the price could range from $2,000 to $6,000 depending on how it’s mounted and installed. For multiple mini-splits, plan to spend up to about $14,500, depending on home size, heat pump capacity, type of heat pump, and how many units you choose.
Ground-source or geothermal heat pumps absorb and release heat underground, where the temperature is a constant 50° F to 60° F all year. They are highly efficient because they don’t have to compensate for big temperature swings the way air-source heat pumps do. But because the heat-exchanging pipes are buried underground (either horizontally or vertically), ground-source systems can be impractical for small lots or those with certain types of soil or landscapes. Ground-source systems can cost from $6,000 to $30,000 or more.
A heat pump’s cooling capacity is measured in British thermal units per hour (Btu/hr.). Btu/hr. can also be expressed in “tons,” with 1 ton equaling 12,000 Btu/hr.
Note that heat pumps need far less capacity to heat a space than a furnace or boiler would because they’re much more energy-efficient. For example, if your home needed a 100,000-Btu/hr. furnace, it may need only a 36,000-Btu/hr. heat pump.
Offline
Like button can go here
