New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2021-01-19 08:24:08
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,163
Subsurface dry ice and liquid CO2
This is an interesting, though somewhat old reference.
https://www.lpi.usra.edu/meetings/geoma … f/7044.pdf
The geothermal gradient of Mars is only 28% of Earth. This suggests that in equatorial and temperate regions, liquid water will not exist above a depth of 4km and above 15km in polar regions. However, liquid CO2 could be stable within a few tens of metres of the surface if it is trapped beneath an impermeable permafrost layer. The paper suggests that this could make drilling quite hazardous. It would also suggest that tunnelling underground could be hazardous unless CO2 pockets are located and drained prior to tunnelling.
On the plus side, stored liquid CO2 would be an excellent power source. And its relatively low pressure and high density would allow it to be piped in plastic and steel pipes. This suggests that early settlements on Mars can direct electric and mechanical power by venting underground CO2 through turbines and allowing it to expand adiabatically. We would probably mount the turbo generator on a trailer, which would be towed to a new site as soon as well pressure declines beneath a critical level. We would connect the trailer to the base via long reels of polyethylene insulated cable.
Looking at the NIST fluid database, liquid CO2 at 217K has internal energy of 80KJ/Kg, whereas gaseous CO2 at the same temperature and 10KPa of pressure has internal energy 400KJ/kg. Low grade solar heat gathered from pipes running along the Martian surface could be used to boil the liquid CO2 raising its temperature to 250K.
https://webbook.nist.gov/cgi/fluid.cgi? … fState=DEF
Last edited by Calliban (2021-01-19 08:46:57)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#2 2021-01-19 18:25:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,604
Re: Subsurface dry ice and liquid CO2
The Mole would have given data with regards to temperature rise with depth along with the latency of shift of it due to water and co2 as you noted which could be. At depth if the reaction of co2 and water due to temperature break down is naturally occurring then this would explain the seasonal venting of methane which is being observed. If the co2 is heated with depth then this is more pressurized and would fall into the yes column for turbine use for sure for power generation once we drill.
Offline
Like button can go here
#3 2021-01-24 17:28:10
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,604
Re: Subsurface dry ice and liquid CO2
Of course our topics as of late are focusing on co2 and its various states of existence on Mars.
From the landers and rovers we can paint a picture of possibilities.
The many images of Mars have shown us frost co2 on the ground, what appears to be brine leaks and water ice just below the surface. The mole may have also shown that the depth of co2 ice could be meters in depth. One would thing that as we do go deeper that we would find liquid co2, water ice and Brine flowing in the deep of mars but until we can go to drill we are left to look at the seismic data for clues.
Offline
Like button can go here
#4 2021-03-31 18:13:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,604
Re: Subsurface dry ice and liquid CO2
Bump for co2 pneumatic tools
Offline
Like button can go here
#5 2023-12-19 15:11:37
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,604
Re: Subsurface dry ice and liquid CO2
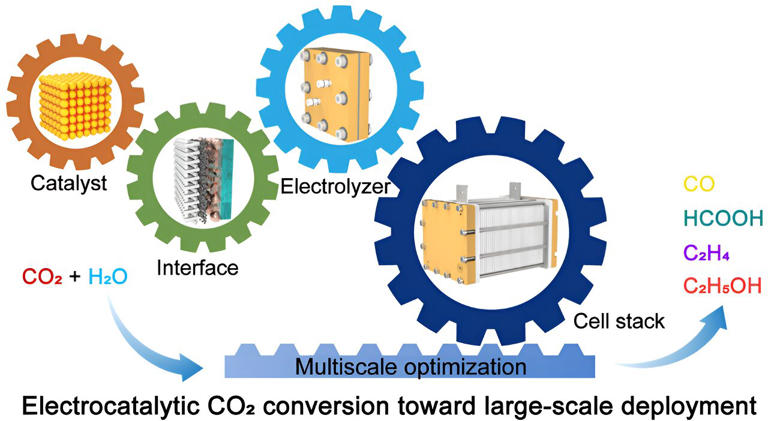
Electrocatalytic CO₂ conversion advancing toward large-scale deployment
Global CO2 emissions continue to grow, reaching 36.1 Gt in 2022, stimulating the implementation of carbon taxes and impacting energy use. The electrocatalytic CO2 reduction reaction (CO2RR) to produce high value-added chemicals and liquid fuels contributes to the construction of green chemical engineering and carbon neutrality.
Decades of research, as well as advances in catalyst design and electrolyzer engineering at the laboratory scale, promise the large-scale deployment of CO2 electrolysis technology.
For practical applications, electrocatalytic CO2RR needs to be executed in a full cell, which unfortunately suffers from low CO2 conversion, poor activity, and stability at the laboratory-scale under industry relevant current densities (> 200 mA cm-2). Consequently, ensuring sufficient production efficiency to reduce product separation costs and electricity consumption is challenging.
To further establish a large-scale CO2 electrolysis system, two dimensions need to be considered: increasing the unit area and quantity of electrode. However, both of them usually bring out a pronounced amplification effect dictated by the complex multi-field coupling, including but not limited to the electric field, reaction field, and flow field. This effect leads to a decrease in the reaction performance and lifetime, ultimately limiting the implementation of industrial CO2 electrolysis with economic benefits.
https://www.sciencedirect.com/science/a … 6723645243
This study offers a comprehensive overview of the multi-scale interlinked research process, with the aim of advancing the commercial application of CO2 electrolysis. The team highlights the challenges involved in realizing high-efficiency CO2 conversion, drawing on the latest research results.
They also examine the future prospects of electrocatalytic CO2 conversion, focusing on the key issues that need to be addressed for its commercial application.
The work points out that while large-scale electrolysis is currently unfeasible, the CO2 electrocatalytic conversion technology and its supporting facilities are advancing rapidly. This progress is accompanied by a decrease in the cost of renewable electric energy.
The researchers emphasize the importance of optimizing the technology from a comprehensive perspective and addressing key issues at different scales involving catalyst, interface, electrolyzer, and cell stack. Focusing on a single aspect is unlikely to achieve the desired results.
Once the roadblocks are overcome, especially in terms of energy conversion efficiency (ECE) and lifetime, CO2 electrolysis will soon become commercially available. However, they also note that the CO2 electrolysis technology is still in its early stage of large-scale applications.
Offline
Like button can go here
#6 2023-12-19 16:01:22
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,163
Re: Subsurface dry ice and liquid CO2
Thanks SpaceNut, interesting. Carbon dioxide is present in seawater at concentration of 1.6% of dissolved gases. So instead of extracting it from air, we would probably degas seawater, as it 40× more concentrated. The energy cost associated with CO2 capture is far more modest if we can extract the CO2 as a biproduct of compressed air energy storage. So we first degas seawater by expising it to a modest vacuum. We then dry the air and compress it until CO2 becomes liquid. Having removed the liquid CO2, we transfer the air to an storage tank in a CAES system.
If we are starting with electricity, then electrolysis is the most efficient way of producing hydrogen. This reacts with CO2 to produce various organic compounds, depending upon the temperature and catalyst used. Integrating the whole process into the electrolysis unit may avoid the cost of an additional chemical reactor. Turning elecrricity into chemical fuels is weak from an overall exergy viewpoint, because only a fraction of stored chemical energy can be recovered as work in an engine. But the fuels produced are at least storable, energy dense and portable hydrocarbons. So the process has that much going for it.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here