New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2023-11-12 08:09:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
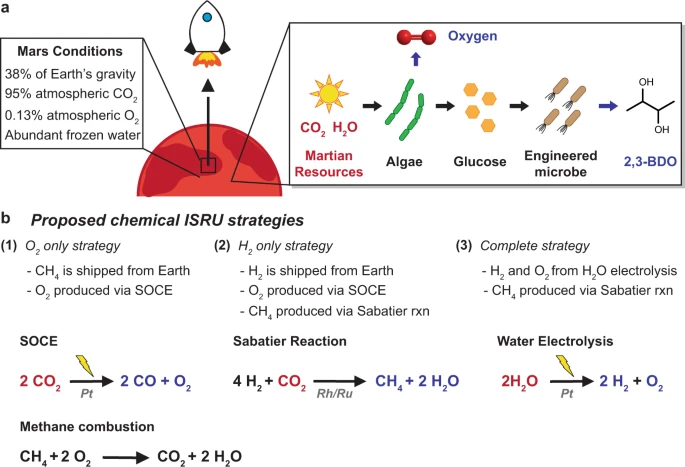
Sabatier Process Deserves it's own topic
As of November 2023, this forum contains 9 pages of topics with posts that refer to the Sabatier process.
It well past time for us to set up a topic dedicated to the process, because it will clearly be a fundamental tool used in many processes at Mars, from production of fuels for transportation or performance of machine tasks such as Earth moving, through many other applications that I suspect are cited in the many topics where the process is cited.
Members are invited to contribute links to scholarly papers on the process, as well as examples of successful use in a variety of industries.
(th)
Offline
Like button can go here
#2 2023-11-12 08:12:06
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Sabatier Process Deserves it's own topic
To start things off, here is a Google Search for Sabatier at Wikipedia...
The process is electrolysis of water by electricity to create hydrogen (which can partly be used directly in fuel cells) and the addition of carbon dioxide CO2 (Sabatier reaction) to create methane. The CO2 can be extracted from the air or fossil fuel waste gases by the amine process.
Sabatier reaction - Wikipedia
Wikipedia
https://en.wikipedia.org › wiki › Sabatier_reaction
About featured snippets
•
Feedback
People also ask
What is the Sabatier theory?
What is the Sabatier principle?
How efficient is the Sabatier reaction?
What is Paul Sabatier process?
FeedbackSabatier principle
Wikipedia
https://en.wikipedia.org › wiki › Sabatier_principle
The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier.Methanation
Wikipedia
https://en.wikipedia.org › wiki › Methanation
The methanation reactions of COx were first discovered by Sabatier and Senderens in 1902. ... It is a means of carbon oxide removal from process gases and is ...Sabatier
Wikipedia
https://en.wikipedia.org › wiki › Sabatier
Sabatier is the maker's mark used by several kitchen knife manufacturers—by itself it is not a registered brand name. The name Sabatier is considered to ...Biological methanation
Wikipedia
https://en.wikipedia.org › wiki › Biological_methanation
Biological methanation is a conversion process to generate methane by means of highly specialized microorganisms (Archaea) within a technical system.Hydrogenation
Wikipedia
https://en.wikipedia.org › wiki › Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, ...Methane
Wikipedia
https://en.wikipedia.org › wiki › Methane
Methane can be produced by hydrogenating carbon dioxide through the Sabatier process. Methane is also a side product of the hydrogenation of carbon monoxide ...Sabattier effect
Wikipedia
https://en.wikipedia.org › wiki › Sabattier_effect
French scientist Armand Sabatier published 26 October 1860 a process of obtaining direct positives (referencing Count Schouwaloff and Poitevin), but ...Talk:Sabatier reaction
Wikipedia
https://en.wikipedia.org › wiki › Talk:Sabatier_reaction
In particular, methane would appear to be an existing method for transporting hydrogen; it transports 25% hydrogen by weight (compare to carbon nanotubes, glass ...Catalysis
Wikipedia
https://en.wikipedia.org › wiki › Catalysis
synthesis gas, which itself is processed via water-gas shift reactions, catalyzed by iron. The Sabatier reaction produces methane from carbon dioxide and ...
Questions & answers
Question
Use the Sabatier process on Earth to generate fuel for Starship?
Answer · 30 votes
It would be energy inefficient and costly to set up. The only reason to do it on Earth would be for environmental reasons; on Mars it would only be used because of a lack of alternatives.
More
Question
Why doesn't SpaceX just build a Sabatier process plant at Starbase?
Answer · 80 votes
I believe that it is the plan to create one, however it is just too early. There are a lot of reasons that they shouldn't. Methane from fossil fuels is significantly cheaper. Starbase is getting grid power from CO2 producing sources today. It would be better for the environment to use any solar power produced at Starbase to sell back to the grid to reduce coal usage in the state. Then from an engineering perspective, the Earth's atmosphere has a very small ratio of CO2 compared to Mars' much higher ratio, and the ambient air temperature is significantly different, and the dust content is also going to be significantly different. However all that said, I think Musk plans to make at least some of his own Methane on site. I would expect he sees Methane as a limited resource in the long run and expects regulation shifts to increase its price over the next 20 years or so. I would also expect that the standard Sabatier reactor, such as the one you've provided is horribly inefficient in some …
More
Feedback
Related searches
sabatier reaction
sabatier process equation
sabatier reaction mechanism
sabatier principle
sabatier reactor
sabatier process efficiency
sabatier reaction enthalpy
sabatier reaction catalyst
(th)
Offline
Like button can go here
#3 2023-11-12 13:10:36
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,220
Re: Sabatier Process Deserves it's own topic
The Sabatier reaction
https://en.m.wikipedia.org/wiki/Sabatier_reaction
CO2 + 4H2O = CH4 + 2H2O
The reaction is exothermic and the highest rate is achieved at 400°C over a ruthenium catalyst. The rate of the reaction will be proportional to the concentration of each reactant. So rate is proportional to the square of pressure. This has economic benefits, as high pressure reactors will be far more compact and will require less ruthenium.
High pressure electrolysis cells produce H2 and O2 at high pressure. This is economically advantageous, because it obviates the need to compress the H2 to high pressure for injection into the reactor. The high pressure H2 from the electrolyser can be input directly into the sabatier reactor. Instead, water is injected at high pressure into the electrolyser. This has lower energy cost, because water is a saturated (non-compressible) liquid. The CO2 can also be injected as a liquid and evaporated by a heat exchanger inside the pressure vessel. By combining HP electrolysis with an HP sabatier reactor and liquid CO2, a very compact and economical plant can be produced.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#4 2023-11-12 18:03:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Sabatier Process Deserves it's own topic
http://www.marspapers.org/paper/Zubrin_1994.pd
Development and Testing of Prototype Sabatier Reactor for Martian In Situ Propellant Production
https://ntrs.nasa.gov/api/citations/201 … hment=true
Another way to put the term into words is ISPP which is in-situ propellant production, a use of mars natural resources so as to not need to bring it from home to mars.
Offline
Like button can go here