New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2022-04-23 07:22:13
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Practical Separation of Molecules for Fun and Profit
This topic is closely related to the one for Separation of Atoms, but it has a far greater and more immediate application.
Separation of molecules is a major industry on Earth, and it will continue to grow as the number of humans increases.
The separation of water molecules from all the other molecules that are present in "natural" water is a major need around the world, and that need will continue to increase.
This topic is available for NewMars members who might like to document known best practice for separation of molecules, or to suggest lines of research to improve efficiency or industrial scale implementation of molecule separation technologies.
It should be kept in mind that energy efficiency is good, but industrial capability is better. If there is need for a metric ton of fresh water, and the energy requirement is X, then a solution that can deliver the metric ton for less than X is good but not sufficient.
What is sufficient is the ability to deliver the metric ton, regardless of the value of X.
The ability to improve on performance by producing the same metric ton with slightly less expenditure of energy is good, provided that the effort needed to exceed X is not in itself so great that X is exceeded.
(th)
Offline
Like button can go here
#2 2022-04-23 07:47:07
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Practical Separation of Molecules for Fun and Profit
The requirement of energy in the separation process is the issue and on earth we combine that with the chemical reaction to liberate elements or molecules.
Early mars is energy intense as we do not have key elements in separated form for use.
For man the first is oxygen as its seems locked up in lots of combinations some requiring more energy than others with each having its own process plus equipment to make use of.
Offline
Like button can go here
#3 2022-04-23 10:43:42
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Practical Separation of Molecules for Fun and Profit
For SpaceNut re #2
Thanks for your contribution to this new topic! While the need for energy to carry out separation of molecules might he self-evident to some, your post is a reminder for all readers that any solution proposed for this topic needs to include specification of the energy required.
I am particularly interested in the specific need for ways to separate H2O molecules from mixtures, in as great a volume as possible, at the lowest expenditure of energy as Nature allows, plus whatever inefficiency we humans inevitably introduce.
I'd like to see the solutions offered in this topic come within 20% of the ideal and achieve 80% of the objective.
The nearby Phoenix water topic is an example of a highly specific need to be addressed, but there are thousands of other locations on Earth where a reliable supply of drinking water is needed in great abundances.
(th)
Offline
Like button can go here
#4 2022-04-23 12:56:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Practical Separation of Molecules for Fun and Profit
water and energy to break it bond into H + O of which the next step is to pressurize or condense to liquid.
https://www.energy.gov/eere/fuelcells/h … ectrolysis
Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700°–800°C, compared to PEM electrolyzers, which operate at 70°–90°C, and commercial alkaline electrolyzers, which typically operate at less than 100°C). Advanced lab-scale solid oxide electrolyzers based on proton-conducting ceramic electrolytes are showing promise for lowering the operating temperature to 500°–600°C. The solid oxide electrolyzers can effectively use heat available at these elevated temperatures (from various sources, including nuclear energy) to decrease the amount of electrical energy needed to produce hydrogen from water.
The electrolysis process uses between 40-50kWh to generate 1 kg of hydrogen. So, using the lower end of that range, the electric power required for 250 kg of H2 is about 10 mWh, which, when scaled up to the full launch amount, is about 10000mWh, or roughly 415 mw of generating capacity working 24 hrs a day is required to supply the hydrogen for a single launch.
Many think that under the ice cap for some craters is a salty water just waiting for the use by man.
This ‘brine electrolyzer’ can mine oxygen, hydrogen from water on Mars
Engineers at Washington University (WashU) in St. Louis
Mars, though, the water contains a fair amount of magnesium perchlorate — salt and their system work better since such high concentrations of salt keep water from freezing on such a cold a planet by lowering the liquid’s freezing temperature to -60 °C.
NASA’s current mandate is to land humans on Mars by 2033. Here, we demonstrate an approach to produce ultrapure H2 and O2 from liquid-phase Martian regolithic brine at ∼−36 °C. Utilizing a Pb2Ru2O7−δ pyrochlore O2-evolution electrocatalyst and a Pt/C H2-evolution electrocatalyst,
To satisfy those same oxygen requirements, the cell active area of the 2.2V brine electrolyzer is .375m and 1.2m^2 respectively.
Offline
Like button can go here
#5 2022-04-23 13:03:39
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Practical Separation of Molecules for Fun and Profit
https://marspedia.org/Sabatier/Water_El … is_Process
https://marspedia.org/Electrolysis
http://en.wikipedia.org/wiki/Electrolysis
https://lunarpedia.org/index.php?title=Water_Splitting
Water Electrolysis
The water electrolysis processing is highly power intensive because of the energy of the bond strength between the hydrogen and oxygen atoms. The electrolysis of water requires a minimum of 237.13 kilojoules of electrical energy input to dissociate each mole of liquid water. Each mole of water gives you 2 grams of hydrogen and 16 grams of oxygen gases. Put another way, commercially available electrolysis systems require about 50 kilowatt-hours of power to produce one kilogram of hydrogen and eight kilograms of oxygen gas from nine kilograms of liquid water.
Offline
Like button can go here
#6 2022-04-23 14:15:39
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Practical Separation of Molecules for Fun and Profit
Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%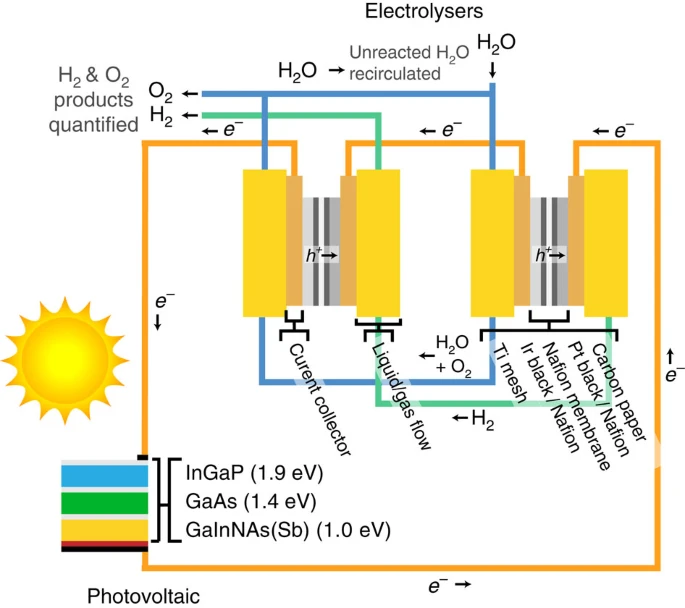
https://www.pnas.org/doi/10.1073/pnas.1900556116
Hydrogen from seawater: Using solar power for electrolysis
The team used coatings nickel-iron hydroxide on top of nickel sulfide, covering a nickel foam core. The nickel foam acts as a conductor, enhancing the transport of electricity to the system.
Without the coating, the anode only functioned for 12 hours in seawater.
“The whole electrode falls apart into a crumble,” said researcher Michael Kenney. “But with this layer, it is able to go more than a thousand hours.”
The team were able to conduct 10 times more electricity through their device than similar systems.
Offline
Like button can go here
