New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2003-08-21 15:27:40
- Bill White
- Member
- Registered: 2001-09-09
- Posts: 2,114
Re: Supercritical CO2 - Useful technology?
Here is one link to a process that uses supercritical CO2 to modify Portland cement into a more useful form.
Using supercritical CO2 in making cement
Because supercritical CO2 also is a solvent, it could also be used to deliver metals, polymers or other materials into cement pores, thereby altering the surface chemistry to prevent penetration of other substances, Jones points out.
Might this process be adapted to make better "Mars-crete"?
In particular it seems that additives can be readily and easily dispersed throughout the cement mixture before curing which might allow "doping" the Mars-crete with useful substances to enhance performance in Marsian construction projects.
Supercritical CO2 can also be used to sterilize stuff without water, dry clean clothes (& dirty diapers?) and extract useful minerals from Marsian regolith. There will be no shortage of CO2 on Mars, right?
Anyone know anything about this subject not readily available on google? I hope to edit and add more links as time allows.
Offline
Like button can go here
#2 2003-08-23 00:15:50
- Shaun Barrett
- Member
- From: Cairns, Queensland, Australia
- Registered: 2001-12-28
- Posts: 2,843
Re: Supercritical CO2 - Useful technology?
Hi Bill!
I don't know any more about the use of supercritical CO2 than you do. Probably less! But I did come across an article somewhere else which dealt with the prospects of using this form of CO2 on Mars because of the Martian atmosphere being almost pure carbon dioxide.
I don't think they mentioned concrete in the article I saw but it seems this process you mention could open up a whole new era of tailor-made concrete with different characteristics for different tasks. It would be interesting to experiment with Martian regolith analogue, introducing various contaminants in various concentrations using supercritical CO2, to see what effect it has on the performance of the finished product.
Who knows what exotic and useful materials might result!
[My only problem is with what passes for Martian regolith analogue. As many here know, I'm not at all sure about this 'peroxides or superoxides in the soil' hypothesis. I believe this is a left-over from a gradually eroding information paradigm about Mars, which was formulated to explain data which are better explained by Martian microbes.
It could be dangerous to do experiments with the current crop of soil analogues, come up with certain results, rely on those results, make plans for construction on Mars based on those results, and then find the real Martian soil is quite different!
Sorry! My mind just went off at a tangent for a while there!!]
![]()
The word 'aerobics' came about when the gym instructors got together and said: If we're going to charge $10 an hour, we can't call it Jumping Up and Down. - Rita Rudner
Offline
Like button can go here
#3 2003-08-23 07:42:47
- Josh Cryer
- Moderator
- Registered: 2001-09-29
- Posts: 3,831
Re: Supercritical CO2 - Useful technology?
Hmm, Shaun, obviously that's why a good settlement is going to need a nice chemistry lab along with it. This way once we arrive we won't have to worry about whether or not our ?one shot only? ideas will work. If the first idea doesn't pan out, try again using a different method.
But Bill, I think it's a wonderful idea, too bad the actual process isn't explained in detail. We need numbers. ![]()
Some useful links while MER are active. [url=http://marsrovers.jpl.nasa.gov/home/index.html]Offical site[/url] [url=http://www.nasa.gov/multimedia/nasatv/MM_NTV_Web.html]NASA TV[/url] [url=http://www.jpl.nasa.gov/mer2004/]JPL MER2004[/url] [url=http://www.spaceflightnow.com/mars/mera/statustextonly.html]Text feed[/url]
--------
The amount of solar radiation reaching the surface of the earth totals some 3.9 million exajoules a year.
Offline
Like button can go here
#4 2003-08-27 20:43:30
- colonist
- Member
- Registered: 2002-03-23
- Posts: 24
Re: Supercritical CO2 - Useful technology?
My company prints a monthly newspaper for the drycleaning industry and I read an article 2-3 months ago on CO2 as the "next miracle drycleaning formula."
From what I read, it seems that the CO2 can be targeted to disolve spesific compounds while leaving simlar ones untouched (organic stains removed whils dyes remain). I am not sure HOW this is done but they seem to have it nailed down prety well. I will see if I can get any info from the next issue of clothesline.
Offline
Like button can go here
#5 2003-08-28 07:34:25
- Bill White
- Member
- Registered: 2001-09-09
- Posts: 2,114
Re: Supercritical CO2 - Useful technology?
Here is another link to spacedaily.
Magnesium burns and is a useful compenent for solid fuel rocket engines. Supercritical CO2 can readily dissolve magnesium found in Mars regolith and thus easily extract and deposit the metal for later use.
Thus, supercritical CO2 can be used to help build rocket engines and also be used to clean the uniforms of the settlers.
How cool is that?
Offline
Like button can go here
#6 2003-08-28 10:54:14
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,321
- Website
Re: Supercritical CO2 - Useful technology?
One of my projects is a high fidelity soil simulant. I am also concerned about the accuracy of Martian regolith analogues, so I'm trying to produce one that is very accurate. The superoxides are produced in a very simply way: put the regolith in a chamber that is exposed to exactly the same atmosphere mixture as Mars, freeze it and reduce the pressure to the same as Mars, then expose it to the same UV light as Mars. That does produce superoxides, and the process is an exact duplicate of Mars conditions, so I believe it is accurate. I don't use anything else to create super oxides.
I'm still working with a geologist to perform a CIPW analysis of results from Mars. I'm synthesising results from Viking, Mars Pathfinder, Mars Global Surveyor, and now Mars Odyssey. There are some surprising results; there is so much water found by Odyssey and clay discovered by MGS that the calculations only make sense if the apatite mineral is completely weathered away into clay. That releases all the chlorine as salt instead of incorporated into minerals. That makes the regolith quite salty. To make the numbers balance I also had to introduce a little hematite, which may not seam surprising at first because Mars is so red, but hematite tends to be formed in hot springs. The evidence of water resolves part of that dilemma. I'm still working on the balance of hydrated minerals; their chemical formula is more complicated so it's not as clear how to balance them. I want results that are perfectly consistent with all results from Mars before making the regolith simulant available.
Offline
Like button can go here
#7 2003-08-28 13:42:27
- Bill White
- Member
- Registered: 2001-09-09
- Posts: 2,114
Re: Supercritical CO2 - Useful technology?
I want results that are perfectly consistent with all results from Mars before making the regolith simulant available
Then, we plant grapes in Robert's soil simulant, make wine and sell it for $500 a bottle to raise money for Mars exploration.
It will probably be lousy wine, but the emotional appeal of "genuine" Marsian wine would be staggering.
Offline
Like button can go here
#8 2003-08-28 16:31:03
- clark
- Member
- Registered: 2001-09-20
- Posts: 6,375
Re: Supercritical CO2 - Useful technology?
Then, we plant grapes in Robert's soil simulant, make wine and sell it for $500 a bottle to raise money for Mars exploration.
I have a recipe for Marsian 3G. :laugh:
Offline
Like button can go here
#9 2003-08-28 16:51:59
- dickbill
- Member
- Registered: 2002-09-28
- Posts: 749
Re: Supercritical CO2 - Useful technology?
The superoxides are produced in a very simply way: put the regolith in a chamber that is exposed to exactly the same atmosphere mixture as Mars
Very interesting. But what do you call Regolith exactly ? I mean, with what do you start : basalts, volcanic stones with added salts or oxydes ?
Offline
Like button can go here
#10 2003-08-28 23:51:12
- Free Spirit
- Member
- Registered: 2003-06-12
- Posts: 167
Re: Supercritical CO2 - Useful technology?
If the process is as easy as the article mentioned, it seems generating water from the regolith could turn out to be the most beneficial use of hypercritical co2. It would beat getting out the witching stick to find big reservoirs of water to tap. Here's the snippet from the article:
Pulling water from rocks will probably have the biggest payoff, at least in the short term, says Debelak. In addition to drinking, "you can split water into hydrogen for fuel, and oxygen for breathing--or as an oxidizer for some sort of engine." Eventually, colonists could set up plants that use CO2 from the martian atmosphere to process hundreds of kilograms of raw material a day.
My people don't call themselves Sioux or Dakota. We call ourselves Ikce Wicasa, the natural humans, the free, wild, common people. I am pleased to call myself that. -Lame Deer
Offline
Like button can go here
#11 2018-12-09 18:24:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,014
Re: Supercritical CO2 - Useful technology?
Just finished fixing topics artifacts....
So getting enough co2 to superheat for a fluid to make materials seems to be quite beneficial
Offline
Like button can go here
#12 2018-12-09 19:39:55
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Supercritical CO2 - Useful technology?
scCO2 is good but how about chemical reduction of carbon dioxide by hydrogen from water splitting to oxalic acid that is a solid. From ethylene glycol, glycolaldehyde, glyoxal, glycolic acid, glyoxylic acid, oxalic acid to two molecules of carbon dioxide, there are six choices of oxidation to be utilized.
Offline
Like button can go here
#13 2022-08-19 20:16:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,014
Re: Supercritical CO2 - Useful technology?
Scientists use supercritical carbon dioxide to power the grid
When it comes to turning turbines, steam is out and supercritical carbon dioxide is in – as demonstrated by Sandia National Labs when it connected a closed-loop system to the local grid, supplying 10 kilowatts of power for nearly an hour. …
Sandia's experimental unit uses carbon dioxide in a supercritical state (pressurized to behave like a liquid and gas) to move a turbine. Supercritical CO2 can get far hotter than steam, and could be far more efficient if scaled to power plant levels and heated via nuclear, solar or fossil fuels.
Ten kilowatts isn't much, around one-third the energy used by the average US home in a day. However, the fact that the lab was able to connect its test loop directly to the grid is a huge deal, said lead researcher Darryn Fleming.
"It took us a long time to get the data needed to let us connect to the grid. Any person who controls an electrical grid is very cautious about what you sync to [it], because you could disrupt the grid," Fleming said.
The Brayton cycle is a thermodynamic alternative to the Rankine cycle, which evaporates water to form steam used to turn turbines.
The Rankine cycle is inefficient and loses a lot of energy turning steam back into water, resulting in only around a third of the power generated being converted into electricity. Per Sandia, Brayton cycles can reach a theoretical conversion efficiency upwards of 50 percent.
n Sandia's system supercritical CO2 is heated, and energy is sent through a turbine. Upon exiting the turbine, the CO2 flows through a recuperator, which cools it before sending it to a compressor. The compressor re-pressurizes the cooled CO2, sends it back through the recuperator to recoup some heat, and then sends it back to the start. A portion of the system's efficiency, the US lab operator said, comes from the recouping process.
For the experiment, Sandia only heated its supercritical CO2 to 600°F (316°C), though fully realized systems would get much hotter. Sandia's diagrams of the system show high-pressure CO2 leaving the heater at nearly double the temperature.
Prior to connecting its Closed Brayton-cycle test loop to the grid in April, Sandia had to find the right control system to regulate its output, and the team found it in an elevator component called a permanent magnet rotor.
The rotors are used to convert potential energy generated by lifting an elevator car into electricity as the car is lowered, and operate on a similar principle to the Brayton cycle test loop Sandia used in its experiment, the lab said.
"This similarity allowed the Sandia team to adapt commercial-off-the-shelf power electronics from an elevator parts company to control feeding power from their test loop into the grid," Sandia said in a statement.
Sandia mechanical engineer Logan Rapp said that coupling the lab's test loop with the elevator bits was a huge step forward, making the line between 10 kilowatts and a megawatt or more "pretty clear." One megawatt could power between 500 and 1,000 homes, and Sandia's industry partners are targeting 1 to 5 megawatts, Rapp said.
With a successful direct-to-grid test behind it, the Sandia team is now working to reach higher temperatures. In 2023 the team plans to build a two-turbine alternator generating system it said will be more efficient, and they aim to demonstrate a 1MW system by fall 2024.
To help make commercial development more appealing, Sandia spokesperson Mollie Rappe told us that Sandia engineers are working on validating parts of the system individually so interested companies can expand on the components as they see fit.
Sandia is no stranger to supercritical CO2 – it's been working on such systems for over a decade. At the time, Sandia said it was leading development, stopping short of describing the supercritical CO2 generators as all but inevitable. Eleven years later, we're starting to inch closer.
Offline
Like button can go here
#14 2022-08-20 04:20:39
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,368
Re: Supercritical CO2 - Useful technology?
I knew that NREL was working on a 10MWe pilot plant with partners from corporate America and academia, and Sandia is on the project as well. There are pilot plants in San Antonio and La Porte.
10-MW Supercritical-CO2 Turbine
Partners in the 10MWe Texas Demo Facility Projects (Toshiba, GE, Texas A&M, and some others aren't mentioned, it would seem):
• Sandia National Laboratories
• University of Wisconsin
• Echogen Power Systems, LLC
• Abengoa Solar
• Electric Power Research Institute
• Barber-Nichols Incorporated
Progress Toward Commercial Deployment of sCO2 Brayton Power Cycles
Offline
Like button can go here
#15 2022-08-20 06:55:48
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,600
Re: Supercritical CO2 - Useful technology?
Question for kbd512 or Calliban ...
Does the 50% efficiency estimate cover the entire plant?
For comparison:
Per Google:
People also ask
How efficient is a fission reactor?
Both nuclear and coal plants show a range of efficiencies. Nuclear plants currently being built have about 34-36% thermal efficiency, while one of the new reactor designs boasts 39%. In comparison, new coal-fired plants approach 40% and CCGT plants reach 60%.
A 50% efficiency for the entire plant would compare favorably with the examples shown.
However, the ** other ** 50% still has to be dumped into the environment.
(th)
Offline
Like button can go here
#16 2022-08-20 07:59:05
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,014
Re: Supercritical CO2 - Useful technology?
What makes up a brayton power cycle engine and can it be altered for a home sized unit that can deliver as note elsewhere the typical 600 kw used in a month.
Offline
Like button can go here
#17 2022-08-20 11:46:27
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,368
Re: Supercritical CO2 - Useful technology?
tahanson43206,
50% efficiency means if 2MWt (thermal power) are pushed through the sCO2 gas turbine, then 1MWm (mechanical power / shaft horsepower) pops out the other end. Modern electric generators or motors (same thing) can be and increasingly are around 95% efficient. In this case, however, I believe what they're noting is that 2MWt (thermal power) results in 1MWe (electrical power). That is a big number in the world of thermal power conversion, especially since it was achieved using such physically small / compact equipment and was a real-world number, not some lab-scale prototype. It says nothing abut the mass of materials required to achieve that efficiency, nor much of anything else, but it's a major accomplishment.
Offline
Like button can go here
#18 2022-08-20 18:34:21
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,600
Re: Supercritical CO2 - Useful technology?
For kbd512 re #17
Thanks for clarifying that the 50% target efficiency is ** just ** for the heat-to-motion component of the plant.
Total plant efficiency would necessarily be less.
Whatever the difference is necessarily goes out into the environment.
I'm interested in this because waste heat from industrial facilities might become significant at some point.
I asked Google for suggestions, and as usual, it found a number of citations to study (if there were time)...
Guidebook for Energy Efficiency Evaluation, Measurement ...https://www.epa.gov › default › files › documentsPDF
This document, Guidebook for Energy Efficiency Evaluation, Measurement, and Verification: A Resource for State, Local, and Tribal Air & Energy Officials, ...
72 pages
People also ask
How do you measure energy efficiency?
How do you measure the energy performance of a building?
How can industrial energy efficiency be improved?
What is energy efficiency benchmarking?
FeedbackMonitoring Industrial Energy Waste - Fluke Corporationhttps://www.fluke.com › ... › Energy efficiency News
Tracing energy consumption. Figure 2. Set up energy logging equipment to measure overall level and quality of consumption and then trace when energy is consumed ...Tools for tracking and benchmarking facility energy performancehttps://www.energystar.gov › industrial_plants › tools-tr...
EPA's ENERGY STAR Portfolio Manager tool helps you measure and track the energy use and greenhouse gas emissions of your commercial buildings and share results.Energy Efficiency in Manufacturing Facilities - IntechOpenhttps://www.intechopen.com › chapters
by GP Moynihan · 2017 · Cited by 6 — This can be achieved by evaluating energy end uses (e.g., lighting, processing equipment, and heating, air conditioning, and ventilation (HVAC) systems), and by ...Energy Efficiency Measure - an overview | ScienceDirect Topicshttps://www.sciencedirect.com › topics › engineering › en...
Energy-efficiency measures create long-lived reduction in electricity use because it is built into the equipment, rather than being dependent on human behavior.Energy efficiency measurement in industrial processeshttps://www.sciencedirect.com › science › article › pii
by E Giacone · 2012 · Cited by 212 — Mathematical process modelling, through statistical analysis of energy consumption data, is used to quantify the specific energy consumption as a function of ...A New Approach to Assessing the Energy Efficiency of ... - MDPIhttps://www.mdpi.com › pdfPDF
by N Verstina · 2022 · Cited by 2 — and sustainable development determine the main trends in the world economy, ... energy efficiency of industrial facilities is of particular ...Benchmarking energy performance of industrial small and ...https://www.diva-portal.org › get › FULLTEXT01PDF
by E Andersson · 2018 · Cited by 47 — Total Energy Efficiency Index for site j. EEM. Energy Efficiency Measure. EEU. Energy End-Use. KPI. Key Performance Indicator.
25 pagesHow energy efficient is your industrial company?https://www.industrialenergyaccelerator.org › general
8. Schedule machinery use – When possible, schedule certain machinery outside of peak hours. Peak hours can constitute up to 30% of a manufacturing facility's ...Metrics for Energy Efficient Buildings: How Do We Measure ...https://www.aceee.org › proceedings › data › papersPDF
by P Fairey · Cited by 9 — measures of energy efficiency such as energy use per unit of conditioned ... EnPIs can be used to benchmark the performance of one facility compared to ...
13 pages
As I suspected, there is a lot more going on at an industrial facility than just the production line (a generator in the present case).
(th)
Offline
Like button can go here
#19 2022-08-20 20:23:39
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,368
Re: Supercritical CO2 - Useful technology?
tahanson43206,
The electric generator is attached to the other end of the sCO2 turbine, so if the turbine is generating 1MW of mechanical power, then a 95% efficient generator is generating 0.95MWe. In practice, they're going to higher temperatures to increase thermal power conversion efficiency beyond 50%, so the 50% overall efficiency claim is real world.
A 250MW sCO2 gas turbine would occupy about the same physical space as a large office desk. It's obviously much heavier than a desk because the turbine casing must be exceptionally strong to contain CO2 at very high pressures, but it's astonishingly small. Similarly supersonic CO2 compressors that were developed with Dresser-Rand go from 10 to 14 compression stages to 2 compression stages. The traditional CO2 compressors fitted to coal-fired power plants shrink from 12-stage monstrosities the size of a small house to something that, once again, easily fits onto the bed of a single semi-truck. Both pieces of turbomachinery easily fit inside a 20-foot ISO container. They're very heavy, no doubt about that, but also exceptionally compact. The diffusion bonded radiators that are about 8X more compact / less material than traditional heat exchanger equipment.
That Toshiba 250MW steam turbine weighed in at 268t. Its paired electric generator weighed 277t. A sCO2-powered equivalent would weigh no more than 26.8t, because it's about 20 TIMES smaller. A 300MW steam turbine is about 20m in length. A 300MW sCO2 turbine is 1m in length. The reason it's only 10X lighter is that there are 2 of those devices for a dual-pass loop. It's still hard to state how much of an improvement that is. The individual pieces of sCO2-powered equipment can be moved with hand cranes. We're going from something drastically larger than the most powerful turbofans used in commercial airliners, as well as heavier than most fully loaded modern twin-jets like the 777 or 787, to something approximately the same size and weight as the more compact turbojets that powered the early jet airliners.
Peregrine Breakthrough sCO2 Gas Turbine Technology
Anyway, the drastic size and weight reduction was why I was so interested in using this equipment on Mars. It's small enough that we could feasibly build a serious power plant capable of running a real city. Maybe we have to go to higher temperatures to achieve the same efficiency, but it's still worth it.
Offline
Like button can go here
#20 2022-08-21 00:47:05
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,368
Re: Supercritical CO2 - Useful technology?
Wright 2MW "Electric Turbofan Engine"
Take note of the coke can for size comparison. That is a seriously compact device, and it uses plain old Copper windings. We have the capabilities to make electric motors / generators much smaller and therefore lighter than they presently are, but keeping them cool is a problem. No matter how efficient, there's always some residual waste heat that must be dissipated. The more compact and powerful the electric motor, the more of a problem it becomes. Using CNT wiring, we could drastically decrease the weight, with the motor casing and rotor being the heaviest parts of the machine, but that weight reduction won't change it's viability as an aircraft power plant.
The same applies to nuclear reactors. We've produced fantastically power-dense reactors in the past for the nuclear aircraft program, and of course, the greatest technical challenge was keeping them cool and identifying new materials that could take the heat and radiation without significant degradation.
The ability to use heat efficiently and the ability to create very power-dense nuclear, sCO2, and electric motor powered devices opens up new applications that were previously impractical, such as nuclear-electric powered aircraft. There's a minimum size for such aircraft, but if we were serious about reducing emissions then we would be actively pursuing such technologies, even if we never put such a machine into service in an aircraft. However, the most obvious first application is an airborne aircraft carrier that can remain airborne for a month at a time, cruising at 500 knots versus 15 knots, able to operate from American shores while reaching the other side of the world in less than a day, so that we do not require fleets of ponderous ships in order to control large swaths of the ocean around us.
Large modern jet engines generate as much horsepower per pound of engine weight as Wright's electric motor, so their claims about producing more power per unit weight are only accurate when compared to jet engines of similar thrust. Most of the cutting edge commercial turbofan engines sacrifice some weight in favor of improved fuel efficiency. A 1% improvement in fuel economy represents serious money.
Wright's claim of simplicity, relative to a gas turbine engine, is almost entirely specious in nature unless you ignore the very real complexity associated with every other aspect of generating the requisite input power to make their motor generate thrust. The electric motor itself is much simpler than a gas turbine engine, but since it must be paired with a gas turbine engine and electric generator, or a fuel cell, or a nuclear reactor, in practice it's only a simpler thrust-generating mechanism that will immediately become much more complex after all the other components to create a functional aircraft are included, as they must be.
Wright's electric motor generates about 6.08hp per pound of engine weight, which is great but definitely not a game changer. Each GE-90 is good for about 110,000hp and it weighs 18,260lbs, so 6.02hp per pound of engine weight.
Wright's assertion about what constitutes a large commercial aircraft must differ from my own, because large commercial aircraft, such as a 737, produce around 30,000hp at takeoff, not 2,700hp.
Wright also conveniently left out the fact that they don't have anything to supply the input electrical power to pair their electric motor with, and even if it was supplied by a gas turbine and turboelectric generator for improved aerodynamics, which is what the rendering of their "futurism plane" shows mid way down the page, it's still burning fuel and then has to supply even more input power due to the inefficiency of having to spin the gas turbine, spin a turboelectric generator, and then spin the primary electric flight motors. Naturally, all of that stuff immediately kills the otherwise superb power-to-weight ratio of the electric motor alone. That's also precisely why nobody does it that way. It's an absurdity.
If nuclear does not supply the input electrical power, then there's no airplane because there's nothing to pair Wright's otherwise great electric motor with that is more efficient than directly coupling the gas turbine to the giant fan that produces thrust. As such, this sort of hyped-up nonsense, and that's all it is, can only be sold to children and people who have no slight clue how aircraft actually work.
We produced a more powerful electric motor. Look at us, we're so green. We spent a ton of money to develop something without a use case. Aren't we great? Okay, sure, but realize you're also a bunch of pretentious clowns with no working aircraft that can use your toy and most of your engineers know good and well that their little creation will never be used for anything other than another tiresome virtue-less signaling stunt to signal to all the ignorant onlookers. It's more vomit-inducing moral preening to demonstrate to everyone else that you lack virtue because you failed to produce a component for a practical working aircraft that can pay for itself. It's like that Solar Electric aircraft with the wingspan of an Airbus A380 that could carry 1 person, the pilot, and cost $70M USD to construct. Wow! What a great use of both public and private money. A functionally useless aircraft that couldn't carry a single passenger, despite having the same wingspan as the world's largest passenger jet. What an accomplishment. False advertising at its finest.
Those 15 minute hop electric aircraft used by Harbor Air in Canada, that fly once per day between recharges, are the closest thing to a practical attempt to produce a fully-electric aircraft that actually pays for itself. Apart from that, it's an utter waste of time and energy.
If Wright Electric instead said they were developing an ultra-lightweight electric generator that could be used to power a city on Mars, a 250MWe generator weighing in at 25,000kg, then they have my undivided attention. That is something humanity actually needs and can actually use without invoking battery technology that doesn't exist and most likely never will exist within our lifetimes. We've developed light weight sCO2 power turbines. We've developed lightweight nuclear reactors for the Aircraft Nuclear Program. We need lightweight electric generators to pair the reactors and power turbines with. The reactor provides the heat, the sCO2 gas turbine converts heat to mechanical power, and the electric generator provides the juice.
Offline
Like button can go here
#21 2022-08-29 10:34:50
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Supercritical CO2 - Useful technology?
Scientists use supercritical carbon dioxide to power the grid
https://www.theregister.com/2022/08/19/ … o2_sandia/
Molecular-level investigation of plasticization of polyethylene terephthalate (PET) in supercritical carbon dioxide via molecular dynamics simulation. People's Republic of China Shanda-Lunan Research Institute of Supercritical Fluid Technology.
https://royalsocietypublishing.org/doi/ … 20606?af=R
Supercritical Fluid Technology
https://www.naturalproductsinsider.com/ … technology
old discussion on new mars
Dry ice pneumatic tool
https://newmars.com/forums/viewtopic.php?id=8815
Offline
Like button can go here
#22 2022-08-29 11:26:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,600
Re: Supercritical CO2 - Useful technology?
For Mars_B4_Moon re #21
Thank you for finding and posting the Sandia link ... I think that the link has been posted before, but seeing it again is helpful.
There is a detail of mechanical design that is not revealed in the simple flow chart at the linked site....
CO2 entering the (heater) from the second cooling output is under about half the pressure it will have when it leaves the heater.
The detail I'd be interested in seeing is how the lower pressure CO2 is admitted to the (heater) component.
I would expect there is a compression subsystem not shown in the simple flow chart.
Otherwise, the higher pressure of the (heater) would flow back through the input port.
If you (or anyone) runs across a report on that detail, I'd be interested in seeing it.
(th)
Offline
Like button can go here
#23 2022-08-29 19:03:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,014
Re: Supercritical CO2 - Useful technology?
A 10 kw unit that would be set up to run for 3 hours would fill the home with sufficient power for the day if its store and used as needed.
Using the heat of a nuclear reactor
https://energycentral.com/c/ec/supercri … loped-smrs
https://www.swri.org/supercritical-carb … er-systems
https://energy.wisc.edu/industry/techno … s-turbines
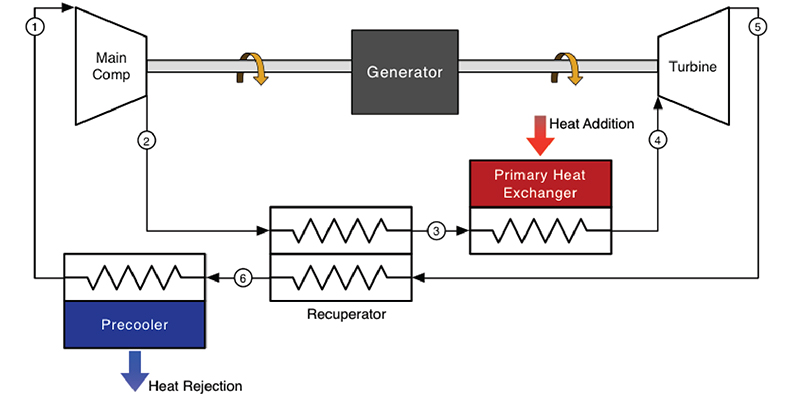
The question of the system is its brought up to pressure much like an AC unit is charged before making use of it. It is a close loop system that will require recharging as it leaks by seals and such over long periods of time.
https://www.energy.gov/supercritical-co2-tech-team
inside workings of the turbine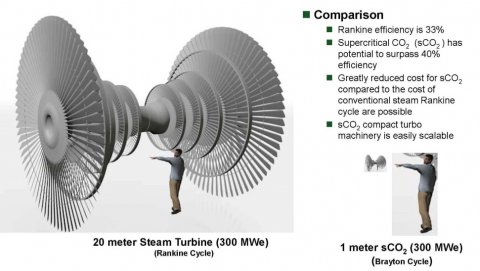
Here is the 10Mw unit that was designed
https://www.energy.gov/sites/prod/files … /55455.pdf
https://netl.doe.gov/sites/default/file … chogen.pdf
Now for mars we would use solar heating to let an internal volume come up in pressure and release it into a small volume as to achieve a higher pressure over time as its transitioned up to liquid each day. The liquid would be pumped into the end pressure tank before making use of it.
Offline
Like button can go here