New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#76 2021-04-06 16:53:32
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
Well mating an RTG unit to the chamber that can uncounted while loading seems practical the get a flow of heat and some electrical power at the same could allow for a semi-automatic production of gaseous co2 from a solidblock fed system.
Offline
Like button can go here
#77 2021-04-06 17:13:17
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For SpaceNut re #76
Thanks for additional support for RTG participation in the process.
For kbd512 ... your post #75 was right at the end of a display series, so I'll have to go back to the bottom of page 3 to see it.
My recollection is that it includes a (to me quite interesting) suggestion for using solar energy to heat salt to melting, and to use that to pressurize a chamber loaded with dry ice for use during the day.
I noted that your vision anticipates live human beings on the job site. That may be necessary. It may prove cost effective.
I'd prefer to see teleoperation for ** all ** such outside operations, with trained staff operating equipment from warm, safe, well lit, comfortable cabins well away from the mayhem of industrial caving operations.
Again, there ** might ** be a cost justification for putting human beings at the cutting face of the Martian terrain, so the possibility definitely exists. That labor is no longer necessary, in the modern age, unless the enterprise is underfunded, and in today's world, that is (apparently) quite common.
(th)
Offline
Like button can go here
#78 2021-04-06 17:52:51
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
The molten salt could be quite useful for a controlled heat flow to the chamber and solar concentration would be some what possible if the scale is correct.
The running on compressed mars air topic link is in the edit to post #72 for a mobile unit movement as well.
Offline
Like button can go here
#79 2021-04-07 07:49:52
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
An Earth-side pressure cooker is an example of a container that is (relatively) easy to load and unload. The target pressure for a tool supply is 7 bar, and a pressure cooker is designed to operate a pressures way below that.
Presto 01781 23-Quart Pressure Canner and Cooker
Visit the Presto Store (Amazon)
4.8 out of 5 stars 9,567 ratings
Brand Presto
Material Aluminum
Color Silver
Capacity 21.77 Liters
Item Dimensions LxWxH 15.4 x 15.1 x 14.8 inches
[PDF] The Polar® Insulated Container System Dry Ice - DACO Corporation
www.dacocorp.com › documents › Bonar › Polar Dry Ice Containers ...
A cubic foot of dry ice pellets weighs approximately 52 lbs. Dissipation ... Kg. 50. Lbs. 110. Cubic Feet. 11. Dry Ice Pellets (lbs). 600. Dry Ice Block. 12. Layers of ...
Per Google a cubic foot is 28.317 liters, so the Amazon example cited above is about 3/4 the size needed for the Pneumatic Tool application.
Also with assistance from Google:
- The operating pressure for PRESSURE COOK, SOUP, STEW, MEAT, CHICKEN, RICE, RISOTTO AND STEAM functions on average is 55-65 kPa which is 7.97 - 9.42 psi. - There is no pressure for the SAUTÉ and BROWNING function as these functions are used without the lid.
Recipes for QT Pressure Cooker with Touch Pad - Bella Housewares
bellahousewares.com › microsite › bella-6qt-pressure-cooker › faq
The Pneumatic Tool application calls for 90 psi, and the tank should be able to handle well over that pressure to be able to maintain 90 psi as a stable working pressure.
This larger canning pressure cooker shows available pressure as 15 psi maximum with volume of 41 quarts ...
All American 941 Canner Pressure Cooker, 41.5 qt, Silver
Visit the All American Store (Amazon)
4.9 out of 5 stars 5,473 ratings
Size: 41.5QT
Color: Silver
Made of durable, hand-cast aluminum with an attractive, easy to clean satin finish; Easy on-off cover; Positive action clamping wing nuts permit easy opening and closing
Sturdy phenolic top handle; Exclusive "metal-to-metal" sealing system for a steam-tight seal; No gaskets to crack, burn, replace or clean
Easy to read geared steam gauge; Automatic overpressure release; Settings of 5 psi, 10 psi, and 15 psi
19 inches high with 15-1/4-inch inside diameter; made in USA
The dimensions of this cooker are in the ballpark of what is needed for the Pneumatic Tool application, but the pressure capability is far short of what is needed.
The tanks included in consumer air pressure systems that exceed 90 psi are NOT designed for heating.
The combination of pressure capability and ability to accept external heating puts the needed boiler into the ballpark where steam locomotives are found.
From Google we have:
In the later years of steam, boiler pressures were typically 200 to 250 psi (1.38 to 1.72 MPa). High-pressure locomotives can be considered to start at 350 psi (2.41 MPa), when special construction techniques become necessary, but some had boilers that operated at over 1,500 psi (10.34 MPa).
High-pressure steam locomotive - Wikipedia
en.wikipedia.org › wiki › High-pressure_steam_locomotive
For this topic, I'd be happy to find a 200 psi boiler that can take externally supplied heat for days, weeks, months and years without losing strength.
And! I'll bet those boilers did not have openings larger than water pipes for intake and steam pipes for output.
The idea of a boiler with a removable lid able to operate at 200 psi and to accept external heating may put this quest into never-never-land territory.
(th)
Offline
Like button can go here
#80 2021-04-07 08:20:45
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
I contacted a manufacturer of steam boilers via online contact chat ... the operator (Pascal) was kind enough to consider the inquiry and offer a phone number to call ...
Visitor34767809: we are working on a concept for operating pneumatic tools on Mars. We're looking for a pressure container that can open fully to admit solid dry ice and then close to hold pressure up to 200 psi while accepting external heat.
Visitor34767809: This is a research investigation. We are exploring a potential market. We have looked at surgical pressure containers and cooking ones. The market potential is both for Mars (long term) and on Earth.
Visitor34767809: For Earth, the potential is to provide gas supply for pneumatic tools in remote locations where on site electricity is not available, or where other factors are in play, such as noise.
You can contact us directly: <number available upon request>
I'll call the company later today.
(th)
Offline
Like button can go here
#81 2021-04-07 13:40:35
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,363
Re: Dry ice pneumatic tool
tahanson43206,
The only way any this Mars Colony is getting built, is if "live human beings" are doing the work.
We need to create a small army of self-replicating robots to build a lunar base. - NASA
You mean "humans"? - Everyone Else
We're going to Mars so humans can live and work there. That's the entire justification for this project. If humans can't live and work there, then there's no point in having an army of self-replicating robots on Mars.
Offline
Like button can go here
#82 2021-04-07 14:39:38
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For kbd512 re #81
I'd like to stipulate here that I greatly appreciate the labor of jackhammer operators, and am glad to see they receive what looks to me like a decent living:
$33,476 a year
As of Mar 27, 2021, the average annual pay for a Jackhammer Operator in the United States is $33,476 a year. Just in case you need a simple salary calculator, that works out to be approximately $16.09 an hour. This is the equivalent of $644/week or $2,790/month.Jackhammer Operator Annual Salary ($33,476 Avg | Mar 2021 ...www.ziprecruiter.com › Salaries › Jackhammer-Operator-...
About featured snippets
•
However, there is NO reason that I can see for anyone paying for personnel to build a base on Mars to skimp on equipment.
If it is absolutely necessary for a worker to don a EVA suit and go fix a broken piece of equipment, that is one thing.
To put a worker at the handle of a jackhammer inside an excavation on Mars is not only unnecessary, it is reckless.
We're not (or at least ** I'm ** not) talking some kind of advanced AI robot worker. I'm talking (and ** have ** been talking ) about extending the teleoperation we already have on Earth, and ** have had ** for decades, to varying degrees.
The drone operator in an air conditioned cabin on an air base in the US is a model for the worker who will be directing machinery on Mars.
The person paid to take on duty on Mars is going to receive a salary a LOT greater than $33K per year, and for that additional remuneration I would expect such a person to have the education and skills necessary to install, operate and maintain a teleoperation capability sufficient to excavate and then refine living spaces for as many people as can be delivered in some reasonable amount of time.
This topic is about developing a Dry Ice Pneumatic Tool. It is ** not ** about robots of ** any ** kind.
I actually logged on just now to retrieve your advice on energy flows, and I got distracted (as often happens) by the interesting posts that have appeared since my last visit.
Thanks for keeping things moving along ... it's been fun seeing all the activity of late, and I've had difficulty keeping up.
(th)
Offline
Like button can go here
#83 2021-04-07 15:06:10
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
As planned, I've printed and studied kbd512's post about the enthalpy of dry ice (and a lot more besides).
I am starting this post with a quote:
4.2 Lbs
Propane weighs 4.2 Lbs per gallon at 60°F, so 100.38 ÷ 4.2 = 23.9 gallons.Customer Questions & Answers - Amazon.comwww.amazon.com › ask › questions
For reasons that don't make sense to me but must make sense to everyone else propane is sold by the gallon.
The calculations needed to determine how much propane is needed are based upon weight, so I asked Google for help with ** that ** value.
As a reminder, the estimate for amount of dry ice needed to run an 8 cfm tool for 60 minutes is 23 kg.
The energy content of propane is a little bit (but not a great deal) less than methane. I'm working with propane because it is sold locally in carry-out containers that would be appropriate for a field test of a dry ice boiler.
As Void would say ... Not Done ...
Returning after home chores ...
The price of propane goes by the gallon (apparently) and locally the price is reported to vary from $2 (US) to $2.75 (US).
I computed a requirement for 7 gallons of propane, working from the figures provided by kbd512 for Methane.
Rounding up, I have an upper bound of $21 (US) for the quantity of propane that would be needed to convert 50 pounds (22+ Kg) of dry ice to useful gas at 90 psi to serve a tool running 8 cfm for one hour.
The total cost for materials then is $70 for an hour of service of this particular tool.
The boiler is currently still a mystery. At the upper end, we have seen a device that can withstand internal pressure of 1900 psi.
That device requires a small crane to lift the top to provide access to the interior, and to secure the lid for a run.
I didn't ask for the price, but expect it would be in the low thousands of USD.
A home pressure cooker with sufficient volume is for sale for (about) $500 (US), but it is only good for up to 15 psi over ambient.
A company that makes boilers for industry in the US invited me to give them a call, so I'm planning to tackle that opportunity in the near future.
***
For the Mars case, the heater would (presumably) be fed by CO and O2. It's not clear to me (at this point) whether it makes more sense to stay with gas under pressure for both, or invest the energy it would take to liquefy them.
Edit#1: here is a bit of information about the US based maker of steam boilers ...
Contact Us | Miura America
https://www.miuraboiler.com/contact
MIURA AMERICA HEADQUARTERS. Miura America Co., Ltd. 2200 Steven B. Smith Blvd. Rockmart, Georgia 30153 U.S.A. Phone: 1-678-685-0929 Fax: 678-685-0930 Email: us.info ...
1. Partner Rep Locator | Miura America
https://www.miuraboiler.com/rep-locator
678-685-0929 FIND A REP Founded in 1927, Miura manufactures modular, on-demand steam boilers with compact footprints, fast response, low emissions, and unparalleled safety, with efficiency, reliability, and safety are at the forefront of our design.
1. Miura America Co., Ltd. Company Profile | Rockmart, GA ...
https://www.dnb.com/business-directory/company...
(678) 685-0929. Company Description. Miura America Co., Ltd. is located in Rockmart, GA, United States and is part of the HVAC Equipment Manufacturing Industry. Miura America Co., Ltd. has 140 total employees across all of its locations and generates $16.94 million in sales (USD). There are 65 companies in the Miura America Co., Ltd. corporate ...
Employees: 70
Phone: (678) 685-0929
Location: 2200 Steven B Smith Blvd, Rockmart, 30153-3662, GA(678) 685-0929 Ref chat with “Pascal” 2021/04/07
Visitor 34767809
(th)
Offline
Like button can go here
#84 2021-04-07 16:18:03
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,363
Re: Dry ice pneumatic tool
tahanson43206,
We pay people to work in mines here on Earth. There's risk involved in everything. Some people have more tolerance for it than others. At the end of the day, prudent engineering and workplace safety considerations will determine where humans should or shouldn't be, on the surface of another planet that's immediately lethal to all human life without providing an Earth-like atmosphere. That said, I quite agree on not skimping on equipment. If we need robots to do excavation work, then we'll bring them, but those also require power, spare parts, maintenance, etc, in addition to their human operators / supervisors.
Offline
Like button can go here
#85 2021-04-07 17:39:47
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For kbd512 re #84
The way we've done things on Earth is a useful model for activities off planet. It is ** also ** an opportunity to improve upon them.
Since we have plenty of time and lots of bright people available, I'd like to think we (humans) can do better on Mars than we have managed to do here.
In my opinion, there should be NO occasion when a human has to be at the tip of the spear unless there is an emergency, which certainly could happen and logic suggests certainly will.
Plenty of people on Earth have gardening as a hobby, and many spend enjoyable and fruitful hours engaged in it, but the greater part of agricultural production is now done (in the US) automatically, and automation is continuing to take over more and more agricultural responsibilities.
What may be fun as a hobby is no longer considered suitable activity for humans to earn a living in developed countries.
(th)
Offline
Like button can go here
#86 2021-04-07 18:47:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
The dry ice to gaseous scco2 does not need to be a single step but can involve items that require less mass and can make use of the liquid stage to aid in control as its pressure is mush lower.
I am thinking of a multi cylinder approach simular to a vehicle engine in a way but with the chambers isolated so that you can cycle them from a common inlet manifold of liquid co2 an provide heat to each cylinders individually so that they vent to a common outlet manifold that has a pressure regulator and sensors on the manifold to sense the pressure and to provide the correct psi to the tool. Each outlet of heated pressure is sensed in the cylinder to allow for the most out of it to the manifold when an electronic valve is opened to allow it to flow out.
Offline
Like button can go here
#87 2021-04-07 19:33:34
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For SpaceNut re #86
Your proposal is interesting, but doesn't it depend upon existence of a liquid phase?
A while back, you posted a chart of the phases of Carbon Dioxide. My recollection is that in the tool scenario, sublimation yields gas directly, with no liquid phase.
In any case, the idea of dividing the production of gas into multiple cylinders to total up to the needed 90 psi is quite interesting!
The problem of building a huge pressure vessel with a removable lid would be exchanged for the problem of managing multiple smaller cavities. I like it! I'm not sure that is what you have in mind, since you didn't provide a sketch, but your words created a working model in ** my ** mind, complete with moving parts. << grin >>.
Speaking of moving parts.... in the original design, there would NOT be any moving parts, except for the pressure regulator mechanism.
Everything else would be fixed in place, while the various gases did their thing.
Is there an advantage to having a mechanism with lots of moving parts? There may be.
If you want a mechanism with moving parts, a traditional piston pump that can compress Mars ambient air would seem a reasonable solution.
The whole point of going with Dry Ice is to eliminate moving parts.
(th)
Offline
Like button can go here
#88 2021-04-07 19:55:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
Moving parts are valves to allow flow of heat tubing that is wrapped around each cylinder were working fluids will depend on quality and quantity of heat available, valve to control when the pressure inside each chamber is great enough to vent to a common manifold that acts as a tank to hold the higher pressure and volume needed to run the tool. Sensors are electrical in nature going to a computer brain to send signals to open and close them as needed to maintain heating flow and pressure. No moving parts as it would be simular to fuel injectors for the liquid control into the chamber.
Liquid co2 is I think at 5 bar with cold mars temperatures
Offline
Like button can go here
#89 2021-04-07 20:10:38
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,363
Re: Dry ice pneumatic tool
SpaceNut,
So, multiple separate CO2 tanks at varying temperatures and pressures?
Why would that require electronic valves?
The pressure regulator for the air tool should be set to whatever it's supposed to be for that specific tool. Some require the same or similar operating pressures, while others require different pressures and flow rates. A small air drill and a paint sprayer may operate at different pressures and require different flow rates. The pressure regulator takes care of the pressure setting and the supply tank either can or can't supply enough volume of air for the intended application. That doesn't mean the tool will be totally unusable, but a drill, for example, may not attain maximum rpm unless the supply can keep up with the demand.
Offline
Like button can go here
#90 2021-04-08 07:37:37
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For kbd512 re #89
I appreciated your conversation with SpaceNut, because while inspired by SpaceNut's vision of multiple tanks instead of one large one, I was perplexed by the question of how to combine them.
Regarding the behavior of tools with insufficient air pressure ... I can confirm from personal experience that when a reciprocating saw is low on pressure, it simply hangs up on the work material. I predict the drill will not just fail to sustain rpm ... it will (most likely) fail to sustain torque needed.
The multiple tank scenario might involve a common bus into which the tanks feed. As I think about this, it occurs to me that since gas pressure will equalize between the tanks automatically and instantaneously (at the speed of sound in the medium) there won't be a difference of pressure between the tanks.
However, and this is the ** big ** advantage of SpaceNut's vision, the cost of each tank is reduced.
What we can't know without further study, is whether the cost of the multiple tank design is ** less ** than that of the big all-in-one design.
In my case (with a 50 pound charge of dry ice) the charge could be distributed over 7 gallon sized tanks instead of one large one.
Those tanks ** still ** have to be designed and manufactured to safely hold 90 psi after being subjected to external heating unevenly applied to the exterior.
As a quote earlier in this topic (from an Internet chat session about the idea of using a propane tank as a steam boiler) reminded readers, the metal that is needed for a steam boiler is chosen to have properties that allow it to flex internally due to uneven distribution of external heating, while maintaining the ability to hold pressure to high values.
(th)
Offline
Like button can go here
#91 2021-04-08 08:05:18
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,363
Re: Dry ice pneumatic tool
tahanson43206,
Why would you want to unevenly heat the tank from the exterior instead?
You have less to bother with if the heating element is inside the tank (a metal pipe inserted through the mouth of the tank). Externally heating the tank with Methane or Propane is a good way to mess with the temper of the steel or Aluminum, which is probably not something you want to do to a pressure vessel. When the heating element (whatever that happens to be) is inside the tank, more of the heat is directed into the dry ice or resultant liquid CO2, instead of into the surrounding environment. There's also less risk of annealing the part of the tank nearest to the flame, since the solid / liquid / gas absorbs more of the heat. Uniform heating is a good thing.
Offline
Like button can go here
#92 2021-04-08 09:09:22
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For kbd512 re #91
Thank you for your (interesting to me for sure) question ...
You used the expression "want to" .... I don't think the expression fits perfectly in this case ....
Human historical precedent goes back hundreds of years, if not thousands if you include cooking by boiling water in the scope.
The design of steam engines (which are fresh on my mind) ** always ** involved external supply of heat.
That said, your introduction of the concept of heating **inside** boiler is interesting on a number of levels.
The solution you devise will necessarily break the integrity of the boiler skin, but the benefit of even distribution of heat energy to the interior of the cavity may well be worth the risk of the additional port.
That said, I accepted your lead and asked Google about precedent for fire inside a steam boiler. Boy! Did I get a quick correction to my (obviously limited) understanding of the design of steam engines.
It may have been a while since I commented here on the forum about the progress of Google's AI ... it is ** really ** coming along.
With only the **simplest** of clues it took "steam boiler with fire inside the cavity" and came back instantly with images and drawings, and also the somewhat discomfiting text from Wikipedia:
Fire-tube boiler - Wikipedia
en.wikipedia.org › wiki › Fire-tube_boiler
This type of boiler was used on virtually all steam locomotives in the horizontal "locomotive" form. This has a cylindrical barrel containing the fire tubes, but also ...
Someone with the background of Calliban would have (of course) known this, but (for whatever reason) I did not.
OK .. reset! .... Steam engines operated at pressures above the 90 psi which is the target for this exercise.
Apparently running fire tubes through the middle of the boiler was not only done but ** common **
In the present instance, taking SpaceNut's idea of multiple boilers instead of one large one, and also taking into account the equal distribution of pressure between smaller boilers due to the known behavior of gases in free space, I am now beginning to glimpse a scenario where combustion gases (propane on Earth and CO on Mars) flow through tubes ** inside ** the boiler(s) to insure an even distribution of heat to the interior of the vessels.
I have received an invitation to call Miura Steam Boilers, but I'm holding off until I have a better sense of the topic. I expect to only get one chance to make a good first impression, and my objective would include attempting to interest their management in supporting the Mars Society by participating in design of equipment for Mars. The company is small ... it only has 70 employees ... but it appears to be doing world-class work (at least that's my impression)
So! Thanks again for Post #91 ....
(th)
Offline
Like button can go here
#93 2021-04-08 09:49:20
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,363
Re: Dry ice pneumatic tool
tahanson43206,
The first ship I was on in the Navy, USS LCC-19 Blue Ridge, used boilers. It's one of two left in the fleet (her sister ship is Mount Whitney) that have boilers. Everything else is diesel, gas turbine, or nuclear powered. Back when I was in, USS CV-63 Kitty Hawk also had boilers, but that was it. IIRC, they de-commissioned JFK around that time, she was of the same class as Kitty Hawk, and also had boilers. That seems like a lifetime ago, but it was only a little over 20 years. Time flies.
We have to break the integrity of a gas storage bottle to put gas or dry ice into and to get gas out of the bottle. I'm talking about inserting the heating element through one end of the bottle. What if we insert the heating element at one end and the valve to get gas out is at the other end (heating element inserted through an orifice at the bottom, control valve at the top; orientation doesn't matter all that much, but still)?
Offline
Like button can go here
#94 2021-04-08 13:00:41
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For kbd512 re #93
SearchTerm:Heating boiler for pneumatic tool gas supply
Hopefully that search term will help to find Post #93 in future: http://newmars.com/forums/viewtopic.php … 87#p178387
Do you have a suggestion where to look for prints of the ship boilers on the vessels you mentioned?
While they were (probably) orders of magnitude larger than even the largest steam locomotives, I'll bet the design principles were similar.
In thinking about this, I realized that images of sailors stoking the boilers of steam powered vessels (such as the Titanic but many others) is probably where I got the idea that heat might have been applied to the exterior of the boiler. I now understand (thanks to your hint and Wikipedia showing reality) that such boilers incorporate pipes to carry hot gases through the volume of the boiler so that heat flows to the water blanket with as much surface area exposed as possible.
How an engineer would design for the peculiar properties of dry ice is something I'm hoping to see, at some point.
***
In the mean time, absent any existing hardware, I'm thinking of investing in a cooking pressure vessel, to experiment with dry ice within the limits of the equipment. It should be possible to measure energy input accurately (electric or propane) (or natural gas since I have a grill but I'm not sure how to measure the flow in that case). If I use a propane tank I can measure the weight before and after a run.
It would be possible to measure the temperature of the gas inside the container. The pressure itself will be revealed by the gauge on the device, and by the safety valve, if it goes off, which is possible.
Edit#1: There are wireless sensors that could be dropped into a pressure cooker to measure the temperature, but I'm not sure how well the RF would make it's way out of an all aluminum Faraday Shield. Perhaps the answer to ** that ** question is to drill a hole for a tight fitting probe, since the pressure is low (only 15 psi greater than ambient)
The time required to move from dry ice dropped into the container to safety valve release could be measured.
The amount of dry ice remaining in the container could be measured, assuming there is a pressure relief valve on a pressure cooker. I'd assume there must be one, but I've never owned one, and I was too young to understand the process when my folks used one for canning.
Thanks for the tip about your first ship ...
USS Blue Ridge (LCC-19) - Wikipedia
https://en.wikipedia.org › wiki › USS_Blue_Ridge_(LC...
USS Blue Ridge (LCC-19) is the lead ship of the two Blue Ridge-class amphibious command ships of the United States Navy, and is the flagship of the Seventh Fleet.
Homeport: Yokosuka, Japan
Namesake: Blue Ridge Mountains
Draft: 28.9 ft (8.8 m)
Status: In active service
History · 1971 · February - WestPac I · Evacuation of Saigon
Impressive she's ** still ** in service !!!
(th)
Offline
Like button can go here
#95 2021-04-08 19:47:30
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
tahanson43206,
Why would you want to unevenly heat the tank from the exterior instead?
You have less to bother with if the heating element is inside the tank (a metal pipe inserted through the mouth of the tank). Externally heating the tank with Methane or Propane is a good way to mess with the temper of the steel or Aluminum, which is probably not something you want to do to a pressure vessel. When the heating element (whatever that happens to be) is inside the tank, more of the heat is directed into the dry ice or resultant liquid CO2, instead of into the surrounding environment. There's also less risk of annealing the part of the tank nearest to the flame, since the solid / liquid / gas absorbs more of the heat. Uniform heating is a good thing.
The issue is the pass through holes required to isolate the metal can from the wired connections under pressure shifts from the minus values to the positive number which is not known for the heating element.
Thats why I was looking at other options such as small expansion tanks that are ganged to produce the volume at pressure from a liquid which is more dense than the dry ice and since its got to be under pressure it better suits the application where in we are making a small shift in temp to get pressures. Dry ice has no pressure in the boiler and must be brought up to pressures at higher temperatures.
Offline
Like button can go here
#96 2021-04-09 05:44:13
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For SpaceNut re #95
Thanks for continuing to think about the Dry Ice heating situation!
For a point of clarification, the CO2 has to be brought up to a temperature closer to what we humans consider "normal" (ie, 70 degrees Fahrenheit or 20+ C)
The tool needs to be kept cool, so it is good to keep the gas temperature low, but it can't be too low either. In your planning for heating (however you decide to do it) please plan to heat your gas to just below 70 degrees at 90 psi.
If you can get a chance to make some sketches of your idea, it would be interesting to see them.
The example of steam boilers (prompted by encouragement from kbd512) reveals that humans long ago decided to run heating tubes through boilers to more efficiently transfer gas heat to liquid water to produce steam.
A complication (pointed out by [Dayton Engineer], is that dry ice is NOT well suited for heating, since (of course) it is not a liquid, and CO2 will go straight from solid to gas without going through the liquid phase in this situation. (as I understand the process).
The only way forward from here (that I can see) is to run an experiment with dry ice in a pressure cooker for canning. The run will be limited to 15 psi over ambient, but it should serve to confirm heating calculations provided earlier in this topic, and more importantly, it should reveal the physical behavior of molecules of CO2 in this situation. Heat will be applied to the base of the container and it will be conveyed to the interior by conduction through aluminum.
On the ** inside ** of the container, the dry ice will be in partial contact with the base of the container.
Some of the heat will flow into the dry ice by conduction, and liberated CO2 will allow more heat to flow via convection. The ratio of conduction to convection should decrease as more CO2 is liberated, until conduction drops to zero as the dry ice is exhausted. At that point, all heating will be by convection until the needed operating temperature (near 70 degrees Fahrenheit) is reached.
In the case of the simple cooker test, I would expect pressure to have set off the pressure relief valve, because (of course) a simple cooker cannot withstand the pressures needed for the tool.
From the experiment, it should be possible to (a) confirm heating calculations (b) determine efficiency of heat flows over time and (c) obtain a sense of the rate of progress of sublimation of the dry ice over time.
(th)
Offline
Like button can go here
#97 2021-04-09 19:11:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
Was thinking about the very thing of how hot it can get but still be safe to operate and to do no harm to the user as well.
here is that triple point chart in Celsius but pressure is in Mpa not PSi or ATM
This one is in ATM
here is the typical co2 tank that would hold the final pressure for the tools to make use of.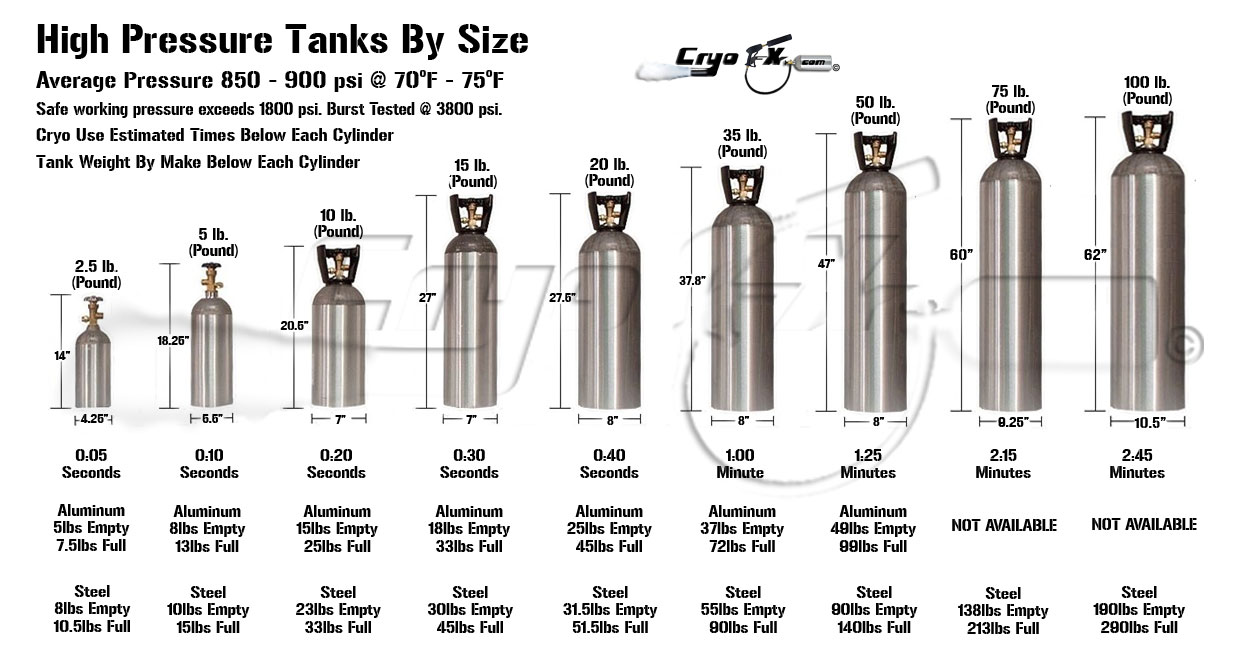
Offline
Like button can go here
#98 2021-04-09 19:35:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For SpaceNut re #97
Thanks for putting those graphs together ... The ATM one was immediately helpful, because the target pressure is 7 ATM.
It appears (I'm hoping for correction if I misunderstand this) that the triple point may occur as a load of dry ice is warmed to 20 C.
However, I'm wondering if this would actually be beneficial?
In a steam engine on Earth, water is adjacent to the hot fire tubes in a boiler (thanks again to kbd512 for direction on that point), but the end result is gas. What it may be impossible to know is the ratio of water to steam that may be present next to the fire tubes. The same may be true in a dry ice boiler.
Perhaps it doesn't matter ... the desired end result is ** all ** the solid converted to gas, and if some of the CO2 passes through a liquid phase I suspect it won't matter because (as your charts both show) CO2 is a gas at the operating temperature of 20 C.
I'm on the verge of feeling confident enough to call Miura ....
I think a working steam engine boiler manufacturer is a much better match with the problem at hand than any of the alternatives I've seen to date.
The quirk of the Mars Dry Ice challenge is the method of feeding raw material into the boiler. Presumably, the smaller the opening the better, but on the ** other ** hand, (remembering caution from SpaceNut and kbd512 about contamination of the Dry Ice feedstock) it seems advisable to design the boiler so the clinkers can be removed, just as they were for hundreds if not thousands of years for coal/wood fired heating systems.
** None ** of the tanks shown in the bottom graphic in Post #97 appears (to my eye anyway) to be designed to open for cleaning.
(th)
Offline
Like button can go here
#99 2021-04-10 08:23:56
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,987
Re: Dry ice pneumatic tool
Dry Ice as a solid has no pressure as its the holding gas at temperature that they are accounting for in the charts that at temperature is able to keep the block solid and is why it sublimes so easily on mars as the atmospheric pressure is so low.
The regulated pressure out to the tool must have a greater pressure contained in the tank to give the desired flow to the tool.
Starting at the 20 Atm and going accross to the 20' C you will see that its going to transition from solid to liquid then to a gas as you cross the -20' C on the triple point line. The triple point of -56.57' C at 5.11 Atm just means you can have slush in the tank but the pressure is not useable and the outlet temperature is to cold for use.
I was looking for the way to be able to open the boiler so as to fill it to keep the pressure for the tool useable as with the single tank approach the tool stops working to fill it. That means a multiple intermediate tank is used to maintain the outlet pressure in a liquid form and with mars temperatures it would stay that way in this tank as a mixture of co2 gas with it. The final tank holds the co2 gas which still can be warmed to boost the pressure and working temperatures for a human used tool. Each tank has a check valve to keep back flow from occurring as the co2 is feed from the boiler to the next tank and so on until it exits the tool.
The boiler takes the most energy to bring the solid up to pressure to feed it into the liquid storage tank with each step takes a lower storage pressure but the same amount to create pressure to make the tool operate.
Offline
Like button can go here
#100 2021-04-10 10:14:06
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,571
Re: Dry ice pneumatic tool
For SpaceNut re #99
Bravo for thinking ahead to the need for continuous service to tool users! I'm thinking of a factory as the most likely setting for this, but it could just as well be robot avatars working in an excavation deep into a scarf.
The ability to switch boilers into and out of the gang of boilers would help greatly!
While the initial testing will necessarily be limited to a single container, the goal of parallel piping for a gang of boilers is attractive!
***
Regarding your 20 ATM ... The target pressure is 7 ATM.
The maximum pressure needed by any commercial pneumatic tool is (unknown to me at present)
I went to Google and found this:
Most tools are rated at 90 or 100 psi, so using 120 psi regularly ensures you will be replacing expensive air tools in half the time you should have to.
Air Tool Pressure + 5 Reasons To Use The Right PSI For Air Tools ...
www.vmacair.com › blog › air-tool-pressure-5-reasons-use-right-psi-air-tools
About Featured Snippets
120/15 >> 24/3 >> 8 ATM maximum at the tool head.
You **might** want to run the boiler at 9 ATM assuming the regulator is good at dropping the pressure to 8, but in any case, this system is NOT going to be operating at 20 ATM. That said, a partial presence of liquid may help with heat transfer. As a reminder [Dayton Engineer] cautioned heat transfer to dry ice may be a challenge, although we won't know until we (someone/anyone) tries it.
(th)
Offline
Like button can go here