New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2020-12-20 11:11:28
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For SpaceNut re #24
Preparation for a Mars University vocational education program needs to start here on Earth.
Design of vehicles capable of manufacture and operation on Mars needs to start here on Earth.
If those (and all other) capabilities are not present in the brains of the settlers, there is vanishingly small chance it will grow there, because survival challenges will overwhelm the settlers.
(th)
Offline
Like button can go here
#27 2020-12-20 15:12:00
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Internal combustion engines for Mars
GW,
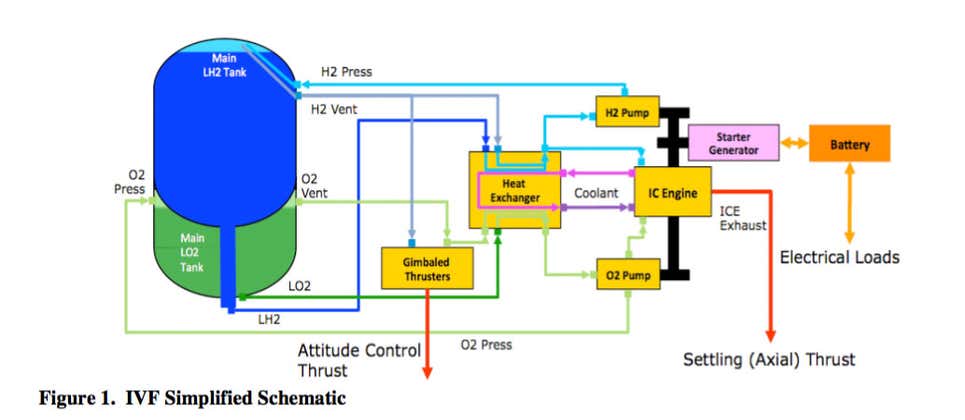
ULA is already running a converted gasoline spark-ignited engine off of O2/H2 boil-off in hard vacuum, since the engine is intended to replace the Lithium-ion batteries and Helium pressurizing tanks on their Centaur upper stage to reduce Centaur's dry mass fraction. If you can run that engine in a vacuum, then you can run a diesel engine in an "almost-hard-vacuum". ULA's IVF (Integrated Vehicle Fluids) combustion engine doesn't require much power, since its goal is to lower the boil-off rate of LH2 (about 24kW max output), which they have achieved, using their converted all-Aluminum inline 6 cylinder racing engine. That engine provides electrical power, cryogenic cooling, pressurization or de-pressurization, and significantly reduces the daily boil-off of LH2. I'm not sure why you think this is impossible, but it's clearly not.
Edit:
Here on Earth we run routinely run diesels on both CNG and LPG, especially Class 8 heavy duty trucks. Most forklifts used in indoor warehouses are either electric or they're LPG powered. I've seen quite a few of those vehicles over the years. The only "new" aspect of this technology set is running pure O2 rather than air (O2/N2 mix) in a semi-closed loop.
Last edited by kbd512 (2020-12-20 15:17:54)
Offline
Like button can go here
#28 2020-12-20 17:12:59
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,083
- Website
Re: Internal combustion engines for Mars
The only reason those engines work (and they do work, I know that) is their fuel and oxidizer gases are supplied at Earthly atmosphere pressures, so that the engine compression can take the cycle pressure to a few hundred psi.
You try that with gases supplied at Martian atmosphere pressures, and you likely won't even get a burn, much less any power out of the engine. The power output from an IC engine depends on the cycle pressure. The compression ratio is a number from 5 to 20. It acts on the feed pressure, which to get a few hundred compressed psi in the cylinders, needs more-or-less Earthly feed pressures.
There is a sharp further increase in pressure as the charge burns, but it is a transient event. The average pressure over the cycle reflects both the compression ratio and the confined-burn rise. That is the so-called "brake mean effective pressure" or BMEP.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#29 2020-12-20 18:11:43
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For GW Johnson re #28
Given your advice about the supply pressure needing to be greater or at least equal to Earth normal (ie, 15 psi) is it correct to plan to maintain the gas supply pressure at 15 psi or greater?
The vehicles operating on Mars (or the Moon for that matter) will carry pressure canisters to hold fuel (assuming it is a gas) and oxidizer (assuming it is oxygen).
I would expect these to be pressurized to the limits of the structural material less some safety margin.
A pressure regulator at the exit from both tanks will be needed to supply the engine.
I understand from your post that Earth normal (15 psi) would be a minimum pressure to supply an IC engine.
Would a greater pressure be even better?
Or is the design of an engine dependent upon the feed pressure?
(th)
Offline
Like button can go here
#30 2020-12-20 18:21:12
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,783
Re: Internal combustion engines for Mars
https://jalopnik.com/a-nascar-team-is-b … 1783198912
NasaSpaceFlight Forum discusion Topic: ULA Innovation: Integrated Vehicle Fluids (IVF) pg3
another forum asking the question What's the point of the ULA's IVF
http://www.ulalaunch.com/uploads/docs/P … t_2012.pdf
http://www.ulalaunch.com/uploads/docs/P … y_2012.pdf
NASCAR’s Roush Is Helping Lockheed and Boeing Build a Rocket Engine
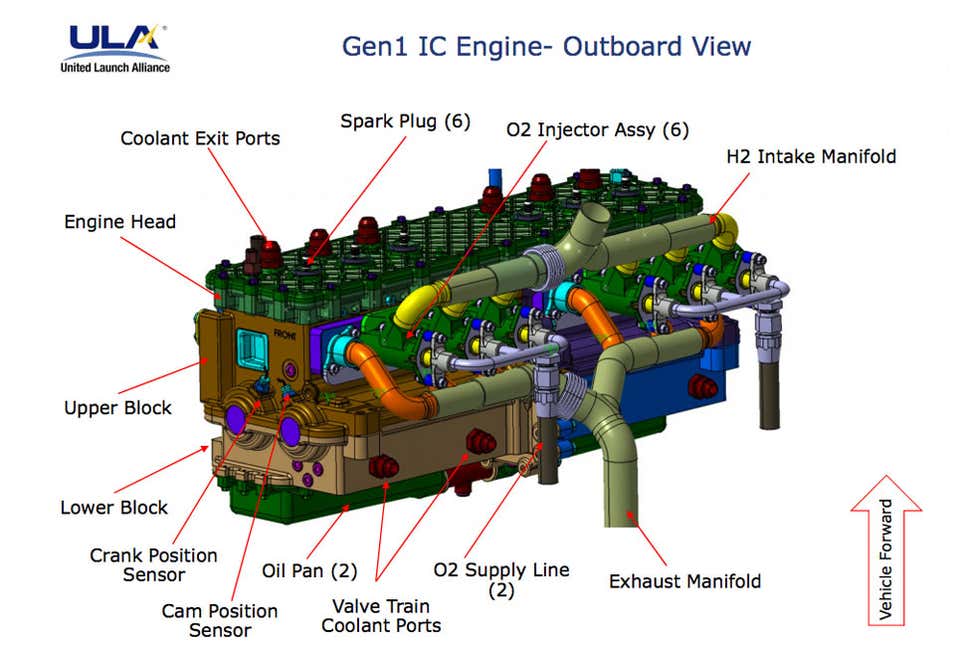
Integrated Vehicle Fluids engine is a 600cc flathead straight-six making 26-horsepower. And by “flathead,” yes, we mean the same engine architecture Henry Ford introduced in the 1932 Model 18. The 27.5-inch-long, 100-pound IVF engine has been designed to use many off-the-shelf components, like its piston rods and spark plugs, and the coil pack is lifted straight from a General Motors 5.3-liter V8.
The IVF engine provides a single power unit that obviates the need for the helium, hydrazine, solar panels, and complex systems layout normally needed in a rocket’s upper stage.

https://en.wikipedia.org/wiki/Advanced_ … lved_Stage

Offline
Like button can go here
#31 2020-12-20 19:01:11
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For SpaceNut re #30
I hope someone has the time to study all those links.
The question at hand is "what pressure is needed for gaseous fuel and for gaseous oxidizer" to insure smooth IC engine operation in a vacuum?
(th)
Offline
Like button can go here
#32 2020-12-20 20:15:20
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,783
Re: Internal combustion engines for Mars
For shuttle External Tank it is was 20 psi for the liquid in the tanks and as it boils the pressure will rise....the next issue is the volume of boiloff rate to supply the intake of the engine with sufficient fuel and oxidizer....
Offline
Like button can go here
#33 2020-12-20 20:34:45
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For SpaceNut re #32
Thanks for that data point. One difference to consider is that the Shuttle External Tank contained liquid oxygen ... the gas pressure would (I presume) have been boil off. The vehicles I ** think ** we are discussing would NOT be using liquid oxygen due to the cost. They ** could ** of course, and for some missions that might be worth while. However, I would expect that for a trip of a few kilometers to buy groceries at the local general store, it should be possible to operate an internal combustion engine with gaseous oxygen and a gaseous fuel of some kind.
I'm trying to find out if it is practical to consider operating an internal combustion engine with gaseous oxygen and gaseous carbon monoxide.
GW Johnson just advised that the pressure at the intake to an internal combustion engine operating on Mars (or presumably in a vacuum) should be at least equal to normal Earth sealevel pressure.
My question flowing from that advice is: What is the ideal pressure for gaseous oxygen and gaseous fuel to provide ideal running conditions for an internal combustion engine designed for Mars?
It may be that GW Johnson's answer will stand uncontested.
In that case, the pressure vessels to be installed on a vehicle designed for Mars that will use an internal combustion engine will need regulators to deliver fuel and oxidizer from the pressure tanks to the engine at Earth normal pressure.
If the fuel is Carbon Monoxide, then the output of combustion will be CO2, which will not be a problem for the people living on Mars.
I'd like to see this topic evolve to develop designs for machines to operate on Mars. Ideally, it would be appropriate for the designs to take advantage of materials available on Mars, and to avoid having to import anything from Earth.
Titan is a potential source of high quality lubricants, or raw material to make lubricants. It would help if a probe designed to explore Titan were designed to look for lubricants as one of its responsibilities.
(th)
Offline
Like button can go here
#34 2020-12-20 20:41:32
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,783
Re: Internal combustion engines for Mars
post 19 and 20 reference the use of lox boiloff for the ivf engine and with that the hydrogen based fuels which go with it.... On mars the storage tanks for the methane and for the oxygen are both in liquified forms so we will have plenty of boil off to make use of...
Now for the co + o designed that engine needs are not the same as an existing engine and needs research and trial and error to see what will work....We think a diesel would work as we would be using a liquid co and lox to make it function at a greater pressure to move the pistons from the ignition of the 2 in the chamber....
I also found a way to reclaim plastics back into gas product on utube videos that I need to search for on a computer so as to provide the link...
Offline
Like button can go here
#35 2020-12-20 21:20:21
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For SpaceNut re ULA's IVF engine ...
https://space.stackexchange.com/questio … cle-fluids
The folks discussing the ULA IVF engine provide some interesting back and forth on the design. A detail I've not been able to find so far is how much pressure the engine sees at the intake manifold. I presume it must be greater than Earth sealevel, (per GW Johnson's advice) but it could be greater, and in any case I would expect the input to be regulated.
***
Thanks for taking up the CO and O engine design. All the references Google supplied about carbon monoxide had to do with its dangers on Earth. On Mars, when operating in an open terrain vehicle, any leakage of Carbon Monoxide would not be a problem because any operator or passenger would be breathing from an oxygen supply anyway.
The energy content of CO is (of course) much less than fuels that require more energy to make.
I can't see why anyone would liquefy Oxygen for a family runabout on Mars. The cost of energy to liquefy Oxygen would be at the expense of something else the family might need, and if the supply is a solar panel I would imagine the family would consider liquefaction a luxury for the uber-rich of the settlement.
This is just a guess right now, but I'll bet that an energy efficiency analysis would reveal that making CO and O from CO2 from the atmosphere to move a ton a kilometer would be more efficient than any other Internal Combustion engine design, by far.
The electricity folks are meeting over in other topics.
(th)
Offline
Like button can go here
#36 2020-12-20 21:48:30
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For SpaceNut re topic
And specifically, use of Carbon Monoxide as the most practical fuel for an IC engine on Mars
The quality of the results I get from Google varies substantially between systems ... the antique I use for much of my work came up with practically nothing useful when I asked if anyone had designed an IC engine to work with Carbon Monoxide...
On a newer machine, the results were ** much ** better .... "charcoal gas" may be Carbon Monoxide .... Here is a first paste of results:
[PDF]The Simple-Fire: An easy way to run an engine on homemade ...
https://www.driveonwood.com/static/medi … e_fire.pdf
Charcoal gas is made from burning carbon in a reducing atmosphere. This gas is then cooled, filtered and mixed with oxygen at the engine. It is sucked into an internal combustion engine where the charcoal gas/air mixture is compressed and burns. Charcoal gas provides about 30% less power than the same engine running on gasoline.
Lean Burn Engine - an overview | ScienceDirect Topics
https://www.sciencedirect.com/topics/en … urn-engine
May 01, 1997 · Carbon Monoxide, Volatile Organic Compounds and Particulates. The emission of carbon monoxide, VOCs and some particulate matter can be partially controlled by ensuring that the fuel is completely burnt within the engine. This is simplest in lean burn engines but conditions within these engines do compromise efficiency. With all engines, careful ..
The estimate of 30% less power than gasoline seems to me to be a bit misleading, because at this point, I haven't read the pdf, and I don't know how the fuels are being measured.
If the issue is horsepower delivered to a load, gasoline and carbon monoxide should be able to provide the fuel.
At this point I'm not clear on whether more or less oxygen is required to achieve a given horsepower.
It would be neat if some of the more knowledgeable members of the forum were to take an interest in this discussion, and perhaps that will happen. In the mean time, if you want to pursue it a bit further, I'm game.
The issue at hand is:
1) What would it take to build an internal combustion engine for Mars that would use CO and O as fuel and oxidizer?
2) For a given horsepower (say 100 for a starting point), what amounts of CO and O are needed for a duration on job. Let's say 1 hour.
3) How much solar energy is required to make the CO and O from Mars atmosphere (cost includes filtering, etc)
4) How much solar energy would be required to liquefy one or both? (I'm guessing it would astonishing)
5) In the absence of liquefaction, how much gaseous CO can be carried in a practical tank at what pressure?
6) In the absence of liquefaction, how much oxygen must be carried in a corresponding practical tank at what pressure?
7) What pressure regulator settings will deliver fuel and oxygen to the engine the optimum levels to insure optimum performance?
That ought to keep someone busy for a half hour at least.
(th)
Offline
Like button can go here
#37 2020-12-21 07:29:44
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Internal combustion engines for Mars
Hopefully we don't go too far off on a tangent here, but some of what is being discussed here is not being explained, so to understand how operating an ICE on Mars necessitates any changes, relative to operating a similar ICE on Earth, let's start with some basic principles and go from there.
ICE = Internal Combustion Engine (I'm sure all of you already knew that)
O/F = Oxidizer / Fuel (ditto)
1. All piston-driven ICEs are intermittently-initiated, explosively-driven heat pumps that use the heat of combustion to rapidly expand a gaseous byproducts from the intake charge and the combustion event, typically with an inert gas such as Nitrogen that acts as a significant component of the working fluid, in order to produce mechanical work. ICEs typically do this by translating the linear movement of one or more pistons moving up and down inside the cylinder bores of an engine block into rotational movement of a crankshaft that the pistons and connected rods are attached to. Neither the pressure of the intake charge, nor the compression ratio of the engine, on their own, can tell you much of anything about the mechanical work that any given ICE can do, nor the relative efficiency with which they can do that work. Other factors weigh heavily into those aspects of how efficiently any given ICE operates. Power losses can come in the form of thermal losses (combustion heat dissipated into the surrounding environment), friction losses (generating waste heat from close-tolerance components such as piston rings moving up and down in the cylinder bores or RPM reduction drive gears in transmissions), pumping losses (the most significant form of losses in prototypical ICEs, as a function of total power loss), and parasitic losses (such as the power used to drive engine accessories such as the water pump, engine oil pump, alternator, or supercharger, if the engine is supercharged).
2. For a given O/F combination (O2/H2; O2/CH4; O2/LPG; O2/Gasoline; O2/Diesel; O2/RP1, etc), there is a specific heat of combustion (thermal power output) that igniting that O/F mixture will produce when mixed in a specific ratio with a particular percentage of oxidizer and fuel. When that mixture is perfectly optimized to produce as much heat as possible, the "stoichiometric ratio" has been achieved. That only tells you what the total thermal energy released through complete combustion of a given mass of that O/F mixture would be, after it’s ignited. That thermal energy then acts upon the resultant combustion byproducts, typically CO2 and water vapor, except in the case of a pure H2 fuel, in which case only water vapor is produced, along with any other species of gas that happen to be present in the combustion chamber, primarily Nitrogen here on Earth. For every O/F combination, there's an upper and lower flammability limit, beyond which ignition of the mixture isn't possible. 100% O2 or 100% H2, for example, are not flammable mixtures, thus combustion of such mixtures is impossible. In Earth-bound ICEs, we commonly refer to O/F mixture ratio the Air/Fuel Ratio (AFR) when discussing the specific mixture being combusted, primarily because most engines use atmospheric air to obtain their oxidizer. However, that "air" from Earth's atmosphere is 21% O2 and 78% N2, on average. If a given O/F mixture was combustible in atmospheric "air", then if all of the N2 was removed from the air, leaving only pure O2, that O/F mixture would still be every bit as combustible, if not more so. At Earth sea level pressure of 14.7psi, of total atmospheric pressure present, only 3.087psi comes from O2, whereas 11.466psi comes from N2.
3. The piston is compressing the intake charge (the O/F mixture plus any inert gases present during combustion) in order to thoroughly mix it, which requires mechanical work to accomplish. The primary objective of compression is to thoroughly mix more oxidizer with fuel inside the cylinder, in order to produce more power from a given cylinder volume. If pure O2 was supplied at Earth sea level pressure of 14.7psi, then almost 5 times as much oxidizer is present to combust the fuel with. While that is fantastic for producing heat, most engines can’t handle producing 5 times as much thermal power in the same volume without serious heat removal modifications. Beyond that, the engine components would also need to be at least 5 times stronger to adequately resist having 5 times as much force applied to them. Pure O2 tends to combust quite rapidly and easily, which is a problem for both spark and compression ignition ICEs that will significantly compress and heat the O/F mixture prior to ignition. To prevent premature ignition, we'll have to dilute the O2 with CO2 captured from prior ignition events.
We don’t have much N2 available on Mars, so we’ll need to use CO2 as a N2 substitute in order to dilute the pure O2. Here on Earth Oxy-Fuel burners in power plants use 95% O2 with CO2 to keep the heat of combustion within tolerable limits. While Nitrogen both heats up during the piston's compression stroke and expands during the power stroke, the Nitrogen naturally present in the air actually absorbs both thermal and mechanical power from the engine since it still takes mechanical work to compress that inert gas. While that's certainly not a bad thing for both mechanical power output and engine longevity, extra input power is required to produce the output power as a result. As GW alluded to, Nitrogen is the compressible working fluid that the heat of combustion expands to drive the piston down the cylinder bore during the power stroke. A good question to ask at this point is whether or not your engine is stout enough to survive 5 times as much heat from combustion or if it will even make as much power without as much compressible working fluid present during combustion. The answer, of course, is probably not on both counts. As such, we will need a replacement working fluid for that Nitrogen. We can use CO2 as a N2 replacement, and we already do, to a limited degree, through exhaust gas recirculation (EGR), although that is not the purpose of EGR in Earth-bound ICEs.
4. If an ICE has a 10 to 1 compression ratio, then the piston's compression stroke takes 14.7psi "air" and turns it into 147psi air, drastically heating it up during that process. Upon ignition of the O/F mixture, the rapid thermal rise may turn the combustion byproducts and inert working fluid into, for example, 1,470psi combustion byproducts. That’s primarily CO2 and water vapor, but the Nitrogen is still along for the ride and also expands drastically, contributing to the total force generated during the power stroke. Given that we can’t practically store enough N2 or CO2 to operate a vehicle engine in an open loop, we need a semi-closed loop to recycle some of the CO2 from prior combustion events, to subsequently serve as an inert working fluid for the next combustion event.
A good example of why the O/F ratio and heat of combustion matters so greatly is graphically illustrated by the difference in O/F ratio of Nitromethane vs Gasoline. It takes about 14.7 pounds of Earth sea level air to completely combust 1 pound of Gasoline, yet it only takes 1.7 pounds of air to completely combust 1 pound of Nitromethane. The power produced from Nitromethane more than doubles the total power output of a type ICE. It adds weight and expense to the engine because the Nitromethane has to be stored onboard the vehicle, but the power increase is also pretty spectacular. In the case of O2 and CH4, 4kg of O2 is required to completely combust 1kg or CH4. If every bit of the oxidizer and fuel had to be stored onboard the vehicle and carried around, that's a pretty dramatic weight difference. Whether or not a Gasoline spark ignition engine was initially lighter than a Methane compression ignition engine of equivalent output, by 200kg or so, is a pretty insignificant data point if both engines will burn 100kg of fuel per hour, for 8 hours straight.
5. This engine I’m proposing is more akin to a coal-fired or gas-fired power plant that captures CO2, rather than a conventional engine. As such, it will certainly weigh more than a conventional open cycle ICE, occupy more volume than a conventional ICE, and consume more fuel per unit of mechanical power output, but it’s being used to power a vehicle that more closely resembles a tank than a passenger car. A 10% power loss to capture 90% of the CO2 output is not a serious problem. Put another way, a 10% increase in fuel burn to produce a given level of power output is not catastrophic to the utility of the engine, which still greatly exceeds the total power output and power-to-weight ratio of a battery powered equivalent as output power level or continuous power requirements increase far beyond what a light passenger vehicle operating on a flat paved highway would require.
Offline
Like button can go here
#38 2020-12-21 09:10:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For kbd512 re Post #
http://newmars.com/forums/viewtopic.php … 41#p175141
SearchTerm:Tutorial on Internal Combustion Engine working principles on Earth plus fuel / oxidizer / energy comparisons
Since this is a new area of study for me, I appreciate the mention of the importance of the dummy component of the gas mixture compressed before ignition.
On Mars, CO2 is available but from a practical point of view, the raw atmosphere can be used as the dummy component. All it requires is filtering to keep airborne sand or other destructive materials out of the combustion chamber.
I did not see simple Carbon Monoxide listed as a fuel, but since it is (to the best of my knowledge) far easier to make for use as a fuel on Mars, I would appreciate it's addition to the topic.
What I understand now is that the ideal mixture of fuel, oxidizer and dummy gas will be regulated by equipment (of some kind) to meet the operating needs of a particular situation (within the limits of the physical engine) while maintaining operating temperatures and pressures at optimum values to preserve the life of the engine.
***
Because my interest here is to find a design for an internal combustion engine that can be created as an indigenous product on Mars, using materials to be found on Mars, and without importing anything from Earth, I am hoping the topic will attract those who will be interested in creating 3D Printer designs for the engine.
At the moment, the only material that (I know of) that exists on Earth capable of service in such an engine is Iconel, but I don't know if the atoms needed to make this material are present on Mars.
There is a detail of manufacture of an internal combustion engine that concerns me at this point ... Piston rings are a very important component of an internal combustion engine, regardless of fuel or oxidizer. Piston rings could be fabricated of Iconel, but (at this point) I have no idea how well they would perform in the intended application.
On Earth, my guess is that fabrication of piston rings is a specialization that has evolved over many decades, and which requires a very large plant and a team of very knowledgeable engineers and technicians to keep in operation. I would guess there may be hundreds of shops that specialize in making piston rings around the planet.
(th)
Offline
Like button can go here
#39 2020-12-21 13:40:13
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Internal combustion engines for Mars
tahanson43206,
SCCO2 = Super-Critical CO2
No, I'm not talking about using atmospheric CO2 in this engine. Once you start doing that, then the effectiveness of the filtration system comes into dramatic play and then you need extra consumables that you have to ship to Mars or otherwise manufacture on Mars. I'm trying to minimize the use of those and build an unbelievably stout compression ignition engine that can operate efficiently over a wide range of output levels. Using atmospheric CO2 would also require an unbelievably power-hungry vacuum pump, as GW already alluded to. I'm talking about using the byproducts of O2 / CH4 combustion to produce a stream of re-compressed CO2, which we re-compress and mix with with the cold O2 input stream, just prior to injection into the combustion chamber, to produce the O2 dilution required, so that we can then reintroduce that mixture for a subsequent combustion cycle / event.
If we were running a combustion engine at constant power output, then I would be advocating for a SCCO2 gas turbine and heater, because that would be far simpler and have far fewer moving components than any piston engine. As previously stated, the problem with gas turbines is that they operate efficiently near their maximum thermodynamic power output level and nowhere else. If you have to run them at idle or partial load, as you so frequently do in vehicle and backup generator applications, then you're guzzling fuel just to keep the gas turbine spinning. A 550hp SCCO2 gas turbine would be truly tiny, something that would fit in the palm of your hand, but running it at less than maximum rated output with desirable fuel economy would be very challenging, to say the least. Perhaps the tiny size of, say, a series of 125hp SCCO2 gas turbines with some creative recompression / re-heating plumbing would justify some manner of gas burner (the burner design very closely resembles a LOX/LCH4 rocket engine's main injector plate, and those get 3D-printed these days to save time and money) and multi-micro-turbine solution for powering vehicles.
I suggest you read the following documents to see what that might look like:
Power generation from coal using supercritical CO2 cycle
Computational Modeling of a Direct Fired Oxy-Fuel Combustor for sCO2 Power Cycles
Commissioning of a 1 MWe Supercritical CO2 Test Loop
Note that the 1MWt Oxy-Fuel combustor can from the second link is 3" in diameter by 10" in length and the O2 is diluted with CO2. The moving components for a 1MWe SCCO2 gas turbine are relatively small, but the radiator / heat exchanger is much larger than the power generation device and electric generator and virtually every component in the gas turbine and heat exchanger is a super grade steel alloy. This may very well prove to be the way to go since a lifetime supply of wear components will neatly fit inside a shoebox. You probably won't be 3D-printing the wear components, but the machinery required to produce replacements would easily fit on a desktop.
Aircraft variable frequency electric generators that generate 90kVA weigh 54kg and are roughly the size of a shoebox.
Aviation Stack Exchange - How much electrical power does one generator produce on a large turbofan?
Please note that the mass of step-up gearing is not needed here since the SCCO2 gas turbine in question will be spinning at a constant speed very near to that of the VFG. Alternatively, we spin up an axial flux DC permanent magnet generator of the type that Magnax (not to be confused with Magnix, which also produces electric aircraft prime movers) produces for wind turbines and electric vehicle and aircraft prime movers, as I originally suggested doing. If it's a stationary backup electric generator, then you probably want the improved efficiency over distant wiring runs on a base that AC provides, at the expense of extra power conversion electronics. If it's an onboard generator supplying power to traction motors a very short distance away, then you probably want a DC generator for simplicity and elimination of potential points of failure. Either way, AC or DC power from a compact unit can be provided. If you're on a highway to hell, then you probably want AC/DC, which can only be provided by one of the greatest rock bands of all time.
Anyway, take a gander at all those high pressure pipes and fittings and tell me if you think it'll really be simpler to maintain that on another planet. You'd best bring a really good plumber with you if this is the way you want to go. The combustor and turbine are simpler than a compression ignition engine, but the total discrete parts count is probably pretty similar, even if the rest of the parts for the SCCO2 gas turbine are simpler to fabricate.
Offline
Like button can go here
#40 2020-12-21 14:23:39
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For kbd512 re #39
This new topic of SpaceNut's is turning into a far more educational exercise than I had expected at the outset.
http://newmars.com/forums/viewtopic.php … 47#p175147
SearchTerm:SuperCritical CO2
***
Regarding on-the-road filtration of Mars atmosphere ....
On Earth, filtration of the atmosphere is a known techology. It is present in every vehicle I have ever seen. Even little lawn mower motors are fitted with air filters.
However, you have alerted the reader of this topic to the potential difficulty of making filter materials on Mars.
That seems to me to be a reasonable caution, and one worth exploring.
However, for the purposes of designing a reliable, inexpensive, easy-to-maintain internal combustion engine solution for Mars, I take your point as leading toward the blending of clean CO with clean CO2 at the manufacturing site, which also produces clean Oxygen.
Since filtering of air is going to be demonstrated by the MOXIE experiment which will be arriving in February of 2021, I am going to be quite interested in (hopefully) learning how the system to be tested actually works in the raw atmosphere of Mars.
***
Now I'd like to invite you to assemble a team to help you design an entire infrastructure for deployment on Mars, using your recommended solution set.
You've been operating here as a Sole Operator for a while, and you have demonstrated you can accomplish a lot in that mode, but your productivity will be magnified if you can enlist support personnel to help with images, diagrams of parts, lists of materials and training needed by operators in the field to set up and to support whatever concept you decide is best for that environment.
Since you are an Administrator, you have the power to modify the recently recovered ID's to bring people you recruit into the forum.
I'd like to see at least ONE (ideally high-value) new member in the forum before this Earth year runs out.
(th)
Offline
Like button can go here
#41 2020-12-21 17:03:48
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Internal combustion engines for Mars
tahanson43206,
The heat of combustion of CH4 is 15.4kWh/kg. The heat of combustion of CO is 2.8kWh/kg. Since we're using nearly pure O2 as the oxidizer, perhaps LCO will be useful for preventing the working components from melting. It would be far more feasible to run nearly pure CO and O2 in a compression ignition engine without serious modifications to contend with the heat of combustion, although reuse of the CO2 working fluid is still required. It would also be far more feasible to run diesel injectors with CO fuel, which would remain in liquid form at the working pressures that modern diesel injectors operate at (25,000psi to 35,000psi, on average). The other feasible consumables reduction I'd want to explore for a Mars diesel engine is eliminating Water / Glycol or Polypropylene Glycol engine coolants in favor of SCCO2, although that would require serious clamp load on the heads and superb seals since both the engine block and heads have to seal nearly perfectly, at 1,200psi (about a third to a half of the transient pressure generated during combustion) for that to work, though it could be a good way to dampen engine vibrations.
Although that embodied energy delta between CO and CH4 fuel is still a pretty difficult problem to overcome, the real question is how many kWh of input power is required to produce CO versus CH4. It takes significantly less power to split CO2 into CO and O2 than it does to produce CH4 using the Sabatier reaction. In fact, concentrated solar energy could produce all of the input thermal energy required. Another obvious advantage is that no precious water vapor is lost to the Martian atmosphere after combustion. H2O tends to be much better at corroding metal engine components than CO or CO2, although pure O2 is also really good at that. Ceramic oxide thermal barrier coatings can passivate the cast iron or steel. The end product is simply the CO2 that you started with, so expelling excess CO2 afterwards is not much of a net resource loss. Both LCO2 and LCO are indefinitely storable at moderate pressures without cryo-cooling, much like LPG, whereas LOX and LCH4 are not. That doesn't mean much, though, on account of how much you need to combust. Even SCCO is nowhere near as dense as cryogenically cold LCO, somewhere between 800kg/m^3 and 850kg/m^3 (about as dense as common grades of kerosene or diesel).
LOX / LCO is also a reasonably good rocket fuel with an Isp in the 280s to 300s range in a vacuum (for comparison purposes, Merlin engines only get 282s of Isp at Earth sea level and 311s in a vacuum; overall, IIRC, LOX/LCO gives you about 1.1 times the bulk density of LOX/LCH4 for the same total impulse, so roughly comparable in terms of propellant volume, but not weight, as LOX/LCO will be significantly heavier even though it also produces more thrust by ejecting heavier molecules; 38% Earth gravity really helps):
Experimental Evaluation of the Ignition Process of Carbon Monoxide and Oxygen in a Rocket Engine
Offline
Like button can go here
#42 2020-12-21 17:22:32
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,083
- Website
Re: Internal combustion engines for Mars
With almost any fuel, you can balance the chemical equation to determine the oxygen required, using molecular weight to get the mole ratio to a mass ratio. Then you can get an Earthly air/fuel ratio by mass from the oxygen/fuel ratio by mass, using the mass percentage of oxygen in air: 0.2315. For Earthly airbreathing combustion, a decent approximation to the lower heating value is air/fuel ratio directly proportional to lower heating value (water not condensed). That approximation does not work for metallized fuels.
At the stoichiometric oxygen/fuel ratio, the flame temperature of most any non-metallized fuel is around 3500-4000 F if burning with air, and 5000-6000 F if burning with oxygen (depends more on initial mixture temperature than fuel type). The marked difference in temperature air to oxygen is the heat-absorbing effect of the diluent gas (nitrogen). The effect of other diluent gases is similar, although the detail numbers shift from fuel to fuel, and diluent gas to diluent gas, primarily due to differing stoichiometric ratios and heat capacities, all else being equal.
For a spark-ignition engine at wide-open throttle, running right at stoichiometry (which presumes in-cylinder mixing due to "squish"), the intake manifold pressure will be right at 1 atm. At part throttle, it is lower than that, due to the pressure drop across the partly-closed throttle plate. It is hardly ever lower than about 20 inches Hg worth of vacuum, which is still very much more than Martian pressure.
For a compression-ignition (diesel) engine, there is no throttle plate, power control is by fuel-air ratio variation. The intake manifold pressure is always near 1 atm, which is why diesel engines have much higher efficiencies than spark-ignition engines (except only at wide-open throttle).
You cannot use Martian atmospheric gases fed at Martian atmospheric pressures as the fuel or diluent in an IC engine. Because the ambient pressure is 6 mbar, compared to Earthly 1013 mbar. You need a compressor to raise them nearer 1 atm "real time", which is the startpoint for how all IC engines operate.
Such a device looks more like a hard vacuum pump than any sort of Earthly compressor. It is far larger than the IC engine it feeds, draws more power than the IC engine can make, and has a throughput far smaller than the massflow through the IC engine. Ugly little facts of life, but there you are.
If you use Martian atmospheric gases, you have to feed them from a pressure tank or bottle, to the IC engine, at around 1 atm pressure to its intake. That means they are pre-packaged, before you ever start the engine. Inconvenient, but that's physics that you cannot fight or ignore.
Diesel engines also have higher compression ratios than spark ignition engines, which is another efficiency-raiser, because it raises the average cycle pressure (BMEP). The incoming charge of only air is compressed very hard, raising its temperature well above 400-500 F. The fuel is sprayed in after this compression, and thus autoignites with the hot air upon contact.
Combustion rate is mixing-rate controlled. Which is exactly why the charge does not explode. Power is regulated by fuel/air ratio: low f/a = low power, high power = richer f/a. Max f/a is smoke-limited to something still well under stoichiometric, for Earthly diesels running on diesel fuel with air.
Spark ignition engines generally mix the fuel into the air before compression takes place. The mixture (NOT plain air) is then compressed, just to a lower compression ratio, so that the compression temperature-rise is less. You have to stay under the autoignition temperature for sure, or else the charge explodes all at once, instead of burning smoothly from the spark location. That would be detonation, which will quickly destroy the engine.
High octane number corresponds to a higher autoignition temperature (usually above 450 F with gasolines). High cetane number = low octane number corresponds to a lower autoignition temperature (usually no more than about 400 F with diesel fuels). The heat of the higher compression and the lower autoignition temperature is how diesels ignite and run at all.
The lesser heat of lower compression, and the higher autoignition temperature, is how detonation is avoided in spark ignition engines. Diesel cannot detonate because combustion rates are mixing-controlled with injection after compression. Spark ignition compresses fuel-air mixture as the charge, so that detonation upon autoignition is the inherent risk. You avoid the detonation risk in diesels by incurring the inefficiency of mixing-controlled combustion.
The higher flame temperatures burning with undiluted oxygen make the cooling problem far worse. Bear in mind that just about the highest energy-conversion efficiency (shaft power divided by the product of lower heating value and fuel flow rate) ever seen out of any IC engine is only about 25%. Most of these fall in the 10-20% range while cruising efficiently, and it ranges down to zero while stopped, idling.
There is a small but significant effect of fuel choice on this efficiency range and on the design of the cooling system. If you burn a fuel that does not soot, you can get about a factor 1.1 increase in shaft output for the same fuel flow rate. The wasted energy (that not made into shaft power) is heat deposited in the hardware. It gets there by convection and by flame radiation.
If your fuel does not soot (and all hydrocarbons do, but alcohols do not), there is almost no flame radiation at all, only convection. I have seen this in automotive and aircraft engines, on the dynamometer, and on the road or in flight. Proving it in aircraft engines was my dissertation.
And I DID prove it! I was 50 years old when I earned that doctorate in engineering. I'm 70 now.
That's as brief as I know how to explain how these things really work.
GW
Last edited by GW Johnson (2020-12-21 17:30:00)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#43 2020-12-21 18:50:12
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For kbd512 re #41
For GW Johnson re #42
It is a rare moment (for me at least) to see the two of you looking intently at the same problem at the same time.
I hope I am up to the challenge of capturing this moment to arrive at a solution that can be recommended for development by future Mars settlers.
I am working purely from memory here, for fear of losing momentum. Therefore, I am sure to have missed something important, or misunderstood something.
1) An internal combustion engine on Mars that uses CO and O2 is feasible
2) Such an engine can operate either with spark ignition or as a diesel engine
3) Intake pressure for both oxidizer and fuel must exceed 15 psi, but may be greater.
4) Liquid CO and Liquid O2 are favored, but an engine will run with gaseous CO and gaseous O2 as inputs
5) The inputs must be prepared ahead of time and made available for use by a vehicle (or fixed stationary engine) as needed
6) Inputs must be as pure as possible given whatever the production circumstances may be
An infrastructure for a Mars IC engine would include:
1) Fixed station to prepare CO and O2 from Martian atmosphere using solar power or other power if available (*)
2) Storage tanks for CO and O2 at the production facility
3) A means of transferring CO and O2 from a manufacturing facility to a vehicle
4) Storage tanks on any vehicle or fixed engine installation
5) Pressure regulators to deliver CO and O2 to the intake at the correct pressure for the situation
As a separate issue .... one of you has proposed using CO2 as a filler gas in the fuel, to play the role that Nitrogen plays on Earth.
That gas needs to be introduced at some point in the manufacturing process, so it is included with the fuel transferred into a vehicle.
I am ** not ** clear on what proportion of CO2 is needed to insure the functions performed by Nitrogen on Earth will be performed correctly on Mars.
It ** seems ** possible (as I attempt to follow the presentations above) that the proportion of CO2 needed in the fuel mix is different depending upon whether the engine to be powered is a spark ignition design, or a diesel.
(*) There can be as many solar powered automated fuel and oxidizer manufacturing stations scattered across the landscape as are needed to support the vehicular traffic anticipated. Fixed sites, such as mining installations, can be supplied by solar powered automated fuel and oxidizer manufacturing stations near the work site. Vehicles such as bulldozers, payloaders, trucks and cranes could all make their way to the refueling stations as needed.
***
If we (contributors to this topic) can agree on the points above or a revised and amended set of those points, then I'd like to move on to considering design of the manufacturing facilities needed on Mars to prepare materials for use in the various components of the system, manufacture of those components, and whatever else needs to be planned that I'm overlooking or simply don't realize are needed.
(th)
Offline
Like button can go here
#44 2020-12-21 19:06:10
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,783
Re: Internal combustion engines for Mars
On earth the oxygen ration is 21% to Nitrogen 67% with 1 volume of fuel of the cylinder size of which the co2 from the atmospher would be run through a turbo charger inlet to raise the volume of co2 for use as one solution with the other being bottled liquid co2 from the factory plant much as the Lox and Lco would be.
A hose form the holding tanks with a valve to turn on the supply and a check valve in the inlet connection to the onboard tanks seem to be the appropriate way to go. A vent valve on the receiving tank to keep internal pressure in check as its filled would also go good with a guage to direct read the pressure as you are filling it. This is what we do for liquid Nitrogen here on earth from a mobile tank system on trucks to a fixed tank at a facility.
Of course the appropriate PPE is a must to do the transfer....
I am wondering if a scaled up moxie would be up to the task for short distance movement in a mobile system all on the same vehicle with solar panels being deployed when stationary to make the fuel for use.
Offline
Like button can go here
#45 2020-12-21 21:13:20
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,083
- Website
Re: Internal combustion engines for Mars
Chemistry:
CO + 1/2 O2 = CO2
Molar oxygen/fuel ratio O/F = (1/2)/1 = 1/2
By mass O/F = (1/2) 32/ (1) 28 = 16/28 = 0.5714
If it was air (synthetic air) 20.9% O2 and 78.1% N2, the oxygen mass percentage is 23.15%, which means the nitrogen mass percentage is 76.85%. The air/fuel ratio by mass is the by-mass (O/F)/.2315 = .5714/.2315 = 2.468.
It is not air, but for the CO fuel to burn about as vigorously (and at the same temperatures) as it would in real air, you use the same mass percentages: 23.15% O2 and 76.85% CO2-as-diluent gas. The same "air"/fuel ratio applies, to get the same mass concentrations: 2.468.
Thus for each pound (or kg) of CO fuel, you need 2.468 lb (or kg) of "air" that is 23.15% O2 and 76.85% CO2. Thus the three stream ratios proportion out as: for 1 lb (or 1 kg) of CO fuel, you will need 0.571 lb (or 0.5713 kg) of O2, and 1.897 lb (or 1.897 kg) of diluent gas CO2.
So that's 1 mass CO fuel + .571 mass O2 + 1.897 mass CO2, for a stoichiometric mixture. Reduce the CO fuel, but maintain the CO2 diluent to O2 ratio, for a lean mixture. Increase the CO fuel, but maintain the CO2 diluent to O2 ratio, for rich mixtures.
I would suggest storing the O2 as LOX in a thermos bottle at modest pressure (1-2 atm), using fed-back heat to support real-time vaporization, and supplying that vapor as part of an inlet stream at just about 1 atm pressure.
I would suggest storing the CO2 diluent as liquid CO2 under rather high pressure, and letting it flash off as you reduce pressure. You then just mix it into the inlet stream with the O2 at about 1 atm pressure. You will need a long duct operating at 1 atm to ensure thorough mixing.
I suggest you store the CO fuel as a liquid under pressure. I know little about its physical properties, but a combination of fed-back heat and pressure drop to 1 atm should gasify it, if gas fuel injection is needed. Inject the liquid CO fuel directly, at something near its high storage pressure, if liquid injection is needed.
If you are operating as a spark ignition engine, inject the fuel as a gas just downstream of the throttle body, into the intake manifold, substantially ahead of the intake valves, to enhance its mixing. Make sure your compression ratio is low enough to avoid compression-heated autoignition (detonation).
If you are operating as a compression-ignition (diesel) engine, do direct cylinder injection of the CO fuel directly from the stored liquid state, at very high pressures. Set your compression ratio high enough to ensure both vaporization and autoignition, with mixing rate-controlled combustion, as the liquid fuel is injected.
The choice of spark vs compression ignition will depend on what that autoignition temperature really is for CO fuel in that kind of "air" with the CO2 diluent. If it's low (say 300-400 F), do compression ignition. If it's high (say 500 F or higher), spark ignition is your best choice.
You will probably need an intake "air" heater (especially on frigid Mars) to get the incoming "air" temperature up to a range where the compressed temperature results look like the experiences with these engines burning Earthly air. Too cold is just too cold, and too hot is just too hot. Something near 30-140 F in the intake manifold (after all the liquids have vaporized) is about right. The colder end of that range is favorable for spark ignition. The hotter end is favorable for compression ignition.
GW
Last edited by GW Johnson (2020-12-21 21:21:09)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#46 2020-12-21 22:15:52
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For GW Johnson re #45
Thank you for this detailed analysis of the CO / O2 (with CO2 diluent) proposal for an Internal Combustion engine infrastructure for Mars.
SearchTerm:CarbonMonoxide analysis of possible use internal combustion engine -
http://newmars.com/forums/viewtopic.php … 67#p175167
Combining the posts from kbd512 with those from GW Johnson, and the overall guidance and motivation from SpaceNut ** should ** lead toward a viable plan for implementation.
I note that in another topic, Calliban has proposed the use of bulldozers to help construct greenhouses for Mars.
It also occurs to me that it might prove advantageous to employ CO/O2/CO2 engine designs to press bricks from regolith (indirectly by driving pumps for hydraulic press equipment).
Edit#1: Overnight additions ...
Elements of an infrastructure (that I can think of right now) include:
Refueling stations at various points in the terrain ... automated, solar powered (except for emergency power), deliver clean CO, O2 and CO2 for customers.
Vehicles of various kinds using engines designed for manufacture on Mars using indigenous materials and solar or nuclear power.
Engines of various sizes for various applications, from small fixed installations for pumping or electrical power supply to ones for bulldozers.
Per advice from kbd512, filters of various kinds to decrease contamination of fuel, oxidizer and diluent to the extent possible. These are to be made from Mars indigenous materials using solar power or nuclear power if available.
Electronic controls for management of flow rates, per GW Johnson, to insure optimum performance under varying conditions and power demands.
Related needs include:
Wheels of some kind, made from indigenous materials. It may prove practical to import raw material from Titan.
Per previous discussion in the forum, tracks for vehicles that need them in the Mars situation. These include guide wheels and track links.
Structural components for vehicles and fixed stations.
Manufacturing facilities ... some of these can be operated at Mars ambient pressures using automation supplemented by remote control.
(th)
Offline
Like button can go here
#47 2020-12-22 15:40:29
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Internal combustion engines for Mars
tahanson43206,
One aspect of using CO that I did not consider is that Carbon Monoxide and Nickel form one of the nastiest little chemicals known, Nickel Tetracarbonyl. That could put a real damper on plans to use CO in a combustion engine, since most of the steel parts contain significant quantities of Nickel to inhibit corrosion and improve operating temperature limits. 30ppm is immediately fatal to humans, so working on high grade steel loaded with lots of Nickel would be a real treat after prolonged exposure to hot flowing CO. The CO will also readily form other highly toxic chemical compounds with metals such as Iron, Manganese, and Cobalt- in other words, pretty much every metal used in high service temperature combustion engine components. Interestingly, a few hundred ppm of Iron Pentacarbonyl reduces the flame speed of a stoichiometric mixture of O2/CH4 by almost 50%, but has never been used as a fire extinguishing agent on account of its toxicity.
Still worth the hazards presented? Maybe. I started looking at why there are no CO-based combustion engines shortly afterwards and why no Nickel-bearing metals or coatings are permitted in labs or fabrication facilities that handle CO. I do know that we switched to electroless Nickel plating after that process became available, on account of how toxic previous Nickel plating processes that used CO were. There's also a significant difference between having some CO gas briefly present in the combustion byproducts leaving through the exhaust and running CO as a fuel. Maybe mixing it with CO2 will reduce the reactivity with the steels and cast irons used, but that really should be tested so that some poor mechanic doesn't merely touch an engine component that was exposed to CO and die shortly thereafter.
In the case of the SCCO2 gas turbine, all parts of the combustion chamber would most likely be high temperature ceramics and the gas turbine operates as part of a closed loop, so there would be far fewer operational issues with using CO as a fuel for that type of gas turbine engine than an all-steel compression ignition engine. Maybe that's why we can't have "simple" piston-driven combustion engines on Mars. If we used the oxidizer and fuel combo that's the least energy-intensive to make (LOX/LCO), then that might limit what types of engines and materials are suitable without producing lethal waste products, since someone will eventually have to clean or repair this thing. If we were working with CH4, this would not be an issue, but then you need a lot more input energy and a hydrocarbon synthesis plant. We might have one of those anyway for making rocket fuel, but storing LOX and LCO in close proximity to each other is less of an explosion hazard. The good news is that with supersonic CO2 compressors and re-heating using the exhaust gas from combustion, most of the pressure loss during expansion through the turbine to generate power can be recuperated to drastically reduce pumping losses associated with re-compression into SCCO2 for the next pass through the loop. The bad news is that this is going to involve fabricating some seriously expensive ceramics, although the parts will be quite small for a vehicle engine.
The LCO storage tank could be ordinary high-Nickel content stainless steel with an appropriate fluoropolymer liner or coating. Alternatively, it could be CFRP with a fluoropolymer coating. Similarly, fluoropolymers like PTFE / Teflon are resistant to pure O2 exposure and not overly-sensitive to impacts while loaded with LOX. For Semiconductor grades of CO gas (99.999% pure), Aluminum rather than steel tanks are used. If I had to guess, that's done to prevent the formation and subsequent transfer of iron-based or nickel-based carbonyls that would interfere with coatings and etching processes used to make microchips. Long story short, the CO needs to be exceptionally pure and stored in vessels that won't form unbelievably toxic surface residues. That said, PTFE coated CFRP tanks would be 1/4 the weight of high strength steel. We could also use an Aluminum-based vacuum thermos as GW suggested doing.
Offline
Like button can go here
#48 2020-12-22 16:42:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,783
Re: Internal combustion engines for Mars
So far none of the fuels/ oxidizers/ or engine types have any issue for outside a protective dome or building for use....So venting of exhaust even inside a building to the outside would also be a none issue.
The only fuel issues are for LCO is breathing hazard, Lox explosiveness in a burning atmospheric leak, LCh4 is only in the presence of oxygen, LCO2 seems no more toxic than CO...so it seems we are a go when we risk mitigation for any of these combinations of use even here on earth with sensors, detectors and venting to keep them from causing any issues.
Thinking of stationary uses the exhaust would be piped to a steel plant for recycling.
Offline
Like button can go here
#49 2020-12-22 18:32:03
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
For kbd512 re #47
First, thank you for your detailed and thorough presentation of the risks associated with Carbon Monoxide, beyond the well known hazard to human health from it's presence in air taken in.
Second ... just plain ... Good Grief!
SearchTerm:CarbonMonoxide hazard when combined with Nickel
http://newmars.com/forums/viewtopic.php … 84#p175184
I sure hope there is at least one more member of the forum membership who is willing to take a look at the situation as laid out by kbd512 here.
What had ** seemed ** like a reasonably practical solution for the special case of Mars Internal Combustion engines, and the infrastructure around them now appears less attractive (at least as I understand the situation now).
SpaceNut has hinted at a possible solution in #48 .... If a system is built to run CO/O2/CO2, and if it is run in the open atmosphere of Mars, well away from any habitat, and if humans always wear their Oxygen breathing apparatus when in the vicinity of the equipment, then perhaps the dangerous compounds that are created will be less of a problem than might otherwise be the case.
Maintenance would pretty clearly have to performed by automation, but that is likely to the the norm on Mars in any case.
Still, it ** is ** disappointing to find these risks added to the scenario.
(th)
Offline
Like button can go here
#50 2020-12-22 18:49:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,135
Re: Internal combustion engines for Mars
Following up on kbd512's caution about Nickel in contact with Carbon Monoxide, I asked Google for the composition if Inconel, which I had hoped would be the 3D printer material of choice for fabrication of machine components on Mars.
I was surprised to learn that Nickel is a major component of the Inconel 718 alloy ...
https://www.azom.com/article.aspx?ArticleID=4459
Chemical Composition of Inconel 718
Typical chemical analysis of Inconel 718.Element
Percentage
Carbon
0.08 maxManganese
0.35 maxPhosphorus
0.015 maxSulfur
0.015 maxSilicon
0.35 maxChromium
17-21Nickel
50-55Molybdenum
2.80-3.30Columbium
4.75-5.50Titanium
0.65-1.15Aluminum
0.20-0.80Cobalt
1.00 maxBoron
0.006 maxCopper
0.30 maxTantalum
0.05 maxIron
Balance
At this point, I am unsure how many of those atom types are present on Mars. A home grown industry based upon 3D Printing using this alloy would never get off the ground if any of those elements are not present.
(th)
Offline
Like button can go here