New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2019-10-25 03:54:12
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,985
- Website
Re: Lunar Nickel Mining
However, they are chemically bonded to other elements. The point about nickel-iron is that is in the form of meteoric dust, so it may well be possible to pan for it using automated crawlers. Also, nickel is a lot more valuable than iron and sodium, so we could actually make a profit by selling it to Terra.
Use what is abundant and build to last
Offline
Like button can go here
#27 2019-10-25 17:41:07
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Lunar Nickel Mining
However, they are chemically bonded to other elements. The point about nickel-iron is that is in the form of meteoric dust, so it may well be possible to pan for it using automated crawlers.
Are nickel and iron available in their metal form?
Offline
Like button can go here
#28 2019-10-25 22:01:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,733
Re: Lunar Nickel Mining
Sure we can hydrogenate the regolith, bake the hell out of it or you can add calcium chloride to it and turn on the current through it to get almost all of the oxygen in the ore with the ore still useable to smelt for the iron or other metals that are in it.
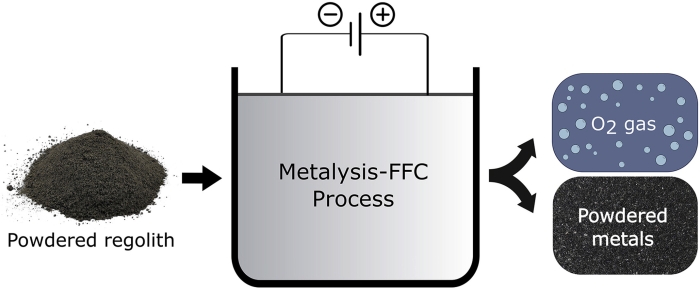
It took around 50 hours to extract 96 percent of the oxygen bound up in the regolith sample, but 75 percent of the oxygen lifted in the first 15 hours.
If time is the only factor for quick an not the tonnage to be moved then the shorter time would yield more oxygen.
Offline
Like button can go here
#29 2019-10-26 04:18:55
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,871
Re: Lunar Nickel Mining
For SpaceNut re #28 ...
Thank you for that impressive link!
This is the most encouraging report I've seen in recent times!
I noted that some of the Oxygen was lost in the process, but I would imagine that if the process is conducted in an enclosed space, the gas would not ** really ** be lost. My guess is that the apparatus for capture of Oxygen used for the study did not capture all that was generated.
I can see an immediate market opportunity, in that launch from the Moon of a variety of materials would be less expensive that would be the case from Earth, and Martian settlers are going to need LOTS of Oxygen. They will be able to choose between Oxygen extracted on site and Oxygen supplied from the Moon. If the energy cost is substantially lower for the Moon product, it will make sense for Martian settlers to use the energy they have available to meet other needs.
(th)
Offline
Like button can go here
#30 2019-10-26 15:04:24
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Lunar Nickel Mining
For tahanson43206 re #29 ...
I can see an immediate market opportunity, in that launch from the Moon of a variety of materials would be less expensive that would be the case from Earth, and Martian settlers are going to need LOTS of Oxygen. They will be able to choose between Oxygen extracted on site and Oxygen supplied from the Moon. If the energy cost is substantially lower for the Moon product, it will make sense for Martian settlers to use the energy they have available to meet other needs.
https://en.wikipedia.org/wiki/Atmospher … rature.svg
Can the Moon and the Mars import nuclear waste from Earth to power Radioisotope thermoelectric generators? That electricity electrolyzes for oxygen and metal. Some amount of oxygen supply settlers, the rest are released on Mars and converted to ozone that is also released on Mars?
The metal are refined for purity. The impure aluminum, calcium, iron, silicon, the stable isotopes from the nuclear waste and the spent fuel from the generators are converted to fluorides. The energy of conversion supplies heat to the lunar settlement. Fluoride of individual element is electrolyzed to the element. Should the fluoride of nuclear waste and spent fuel be treated separately from that of the impure?
A third interesting point is that
https://en.wikipedia.org/wiki/Fission_p … _of_U-235)
Can Al, Ca, Fe, Si be used in artificial nuclear transmutation of nuclear waste on the Moon? Say Iodine-129,
https://en.wikipedia.org/wiki/Isotopes_of_iodine
53I129 + 13Al27 = 66Dy156 (observationally stable)
or I129 + H-1 (possibly from cosmic ray) = Xe-129 (released to be part of lunar atmosphere?)
Or Tc99+14Si28 = La127 (t1/2:5.1min) Ba127 to (12.7mins) Cs-127 (6.25 hours) to Xe-127(36.345day) to stable I-127 (for medical use of iodine for the settlers. So about more than 1 year after Tc-99 is imported to moon, there'll be some iodine for medical use.
Last edited by knightdepaix (2019-10-27 23:17:21)
Offline
Like button can go here
#31 2019-11-11 15:39:59
- Grypd
- Member
- From: Scotland, Europe
- Registered: 2004-06-07
- Posts: 1,879
Re: Lunar Nickel Mining
Can I put my bit in.
Nickel is magnetic so are a lot of the metals we find of interest. still we can use a mass spectrometer to look for more interesting metals like the PGM class of metals.
Chan eil mi aig a bheil ùidh ann an gleidheadh an status quo; Tha mi airson cur às e.
Offline
Like button can go here
#32 2019-11-12 10:11:51
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,985
- Website
Re: Lunar Nickel Mining
Any nickel-iron dust we extract from the regolith will be alloyed with a lot of other valuable metals. Which may or may not be more valuable than the nickel itself.
Use what is abundant and build to last
Offline
Like button can go here
#33 2019-11-15 16:35:05
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Lunar Nickel Mining
Sure we can hydrogenate the regolith, bake the hell out of it or you can add calcium chloride to it and turn on the current through it to get almost all of the oxygen in the ore with the ore still useable to smelt for the iron or other metals that are in it.
https://www.sciencealert.com/images/2019-10/process.jpg
It took around 50 hours to extract 96 percent of the oxygen bound up in the regolith sample, but 75 percent of the oxygen lifted in the first 15 hours.
If time is the only factor for quick an not the tonnage to be moved then the shorter time would yield more oxygen.
Any nickel-iron dust we extract from the regolith will be alloyed with a lot of other valuable metals. Which may or may not be more valuable than the nickel itself.
Can that metalysis process be used to separate nickel and other metals as they are electrolysed on the Moon? I meant not electrolysing every particles of regolith but only to get the metals and some elements out.
Offline
Like button can go here
#34 2019-11-15 16:46:27
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,733
Re: Lunar Nickel Mining
I would think that spin seperation by mass would be a good start to material type, a magnet would be another and then just simply melt them....
Offline
Like button can go here
#35 2019-11-19 16:07:31
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Lunar Nickel Mining
Nickel and Iron can be extracted and redeposited using Carbon Monoxide. You don't need to electrolyse them.
Offline
Like button can go here
#36 2019-11-19 18:24:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,733
Re: Lunar Nickel Mining
Much like earlier you have oxide type materials to seperate for use of co.
http://greenrevolution.org.in/mining/se … l-ore.html
http://greenrevolution.org.in/mining/se … l-ore.html
http://www.mineselect.com/product/nicke … ssing.html
https://www.whitehillstree.com/2018-Dec … l-ore.html
Whats the source of co on the moon?
Here is a reason to mine coal on earth to ship to the moon...
https://file.scirp.org/pdf/IJNM_2016041916153430.pdf
Making Ferronickel from Laterite Nickel Ore by Coal-Based Self-Reduction and High Temperature Melting Process
https://file.scirp.org/pdf/JMMCE_2016110417085843.pdf
Thermodynamics of the Reduction Roasting of Nickeliferous Laterite Ores
Offline
Like button can go here
#37 2019-11-20 11:53:14
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Lunar Nickel Mining
This process used Laterite ore, which you wont find on the Moon, although it might work on a different ore which you might find. It also consumed Calcium Fluoride (fluor spar) and Calcium Hydroxide (slaked lime) and coal all of which are going to be difficult to ship in industrial quantities.
Offline
Like button can go here
#38 2019-11-20 12:15:41
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,985
- Website
Re: Lunar Nickel Mining
The Mond process uses carbon monoxide to separate iron and nickel. It's the obvious choice for extracting it from meteoritic iron.
Use what is abundant and build to last
Offline
Like button can go here
#39 2019-11-20 16:03:08
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,733
Re: Lunar Nickel Mining
CARBON ON THE MOON: 60% obtained from the Apollo
Origin of dark color on the Moon surface has been explained by significant carbon content supplied from impact quenched process, based on data of the Apollo rocks, lunar meteorite, and artificial laser sputtering experiment, and comparison with representative Earth's rocks, where the Moon impacted surface is considered to be carbon-bearing dark ...
Terraformer's Mond Process is meantioned at this source
Carbon Economy
While the equipment can and will be designed to recycle carbon monoxide freed from the carbonyl molecules in this vapor deposition process, process losses to the exterior will inevitably occur. Because the vacuum of the lunar environment will cause near instant disassociation of escaped Fe(CO)5 and Ni(CO)4, that carbon monoxide will be lost.
https://ntrs.nasa.gov/archive/nasa/casi … 009304.pdf
LUNAR RESOURCES: THEIR VALUE IN LUNAR
https://core.ac.uk/download/pdf/52713609.pdf
Carbon in the moon
https://www.lpi.usra.edu/decadal/leag/YasMiura.pdf
LUNAR FLUIDS FROM CARBON AND CHLORINE CONTENTS OF THE APOLLO LUNAR SAMPLES.
Offline
Like button can go here
#40 2019-11-21 14:44:08
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Lunar Nickel Mining
Without an atmosphere, meteorites will impact directly on the surface melting rocks and themselves. Your problem then becomes one of extracting useful minerals from frozen impact melts. Maybe from glasses like those observed by the Chinese lander. In such deposits the FE and NI will likely be present as oxides, silicates, aluminates and so on. On a body without an atmosphere there is little chance of finding a metallic meteorite. One was reported as having been found on Mars and several have been found on earth, for example the Cape York meteorite which was stolen from the Inuit.
Offline
Like button can go here
#41 2024-07-09 14:52:08
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,985
- Website
Re: Lunar Nickel Mining
Proposal to extract water from regolith by first sifting out the ice grains --- https://www.nasa.gov/general/aqua-facto … xtraction/
They claim it to be very energy efficient compared to alternatives, and mention the 1% by weight free metal that could also be removed alongside it. Given concentrations in meteorites, each cubic metre of regolith could be worth $10-20 in nickel. $100 worth per square metre of Luna, and the rock has already been comminuted by billions of years of meteors. The regolith is a pretty reduced environment, so it's unlikely to be oxidised -- doesn't seem to be in samples that have been returned. If processing is straightforward, we could build up quite good nickel stocks.
Slow deposition can add up to vast quantities, if it happens over a long enough (billions of years) timescale. Luna has wealth just sitting there.
Use what is abundant and build to last
Offline
Like button can go here
#42 2024-07-09 16:57:20
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,206
Re: Lunar Nickel Mining
Meteoritic iron powder also contains about 1% cobalt by weight. Another valuable export material. The iron also contains 20ppm (by weight) platinum group metals. Every cubic metre of asteroid iron contains some 158 grams of pt group metals. There may be other valuable elements within this material.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#43 2024-07-10 15:20:29
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,206
Re: Lunar Nickel Mining
This link contains a trace element inventory for metallic meteorites that have fallen to Earth.
https://www.pnas.org/doi/suppl/10.1073/ … 1.sapp.pdf
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#44 2024-07-10 17:58:16
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 22,871
Re: Lunar Nickel Mining
For Calliban re #43
Thanks for that ** impressive ** paper.... the team really worked on that collection of data.
It will take a serious reader some time to absorb all that data. On the other hand, the graphs that are included should help.
If any NewMars member has a bit of time to try to summarize key findings, I (for sure) would appreciate it.
(th)
Offline
Like button can go here