New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2019-01-21 12:26:09
- Void
- Member
- Registered: 2011-12-29
- Posts: 8,923
Atmospheric Resources Methods of Life Support.
I believe that I have stumbled upon a set of references that if taken together point to a potentially good method for Mars.
This topic will also intersect several others such as "Atmospheric Separations" and others.
Here are the two references:
https://techxplore.com/news/2019-01-nor … nergy.html
https://www.lpi.usra.edu/meetings/round … f/6036.pdf
...…
https://techxplore.com/news/2019-01-nor … nergy.html
We know that there is sandstone on Mars, at least near the equator. I suspect that the low basins have it as well, but that it may be covered by more recent sedimentations, such as ice and dust.
Quote:
North Sea rocks could act as large-scale renewable energy stores
by University of EdinburghCredit: CC0 Public Domain
Rocks in the seabed off the UK coast could provide long-term storage locations for renewable energy production, new research suggests.An advanced technique could be used to trap compressed air in porous rock formations found in the North Sea using electricity from renewable technologies.
The pressurised air could later be released to drive a turbine to generate large amounts of electricity.
Using the technique on a large scale could store enough compressed air to meet the UK's electricity needs during winter, when demand is highest, the study found.
The approach could help deliver steady and reliable supplies of energy from renewable sources—such as wind and tidal turbines—and aid efforts to limit global temperature rise as a result of climate change.
However, the amount of energy produced by many renewable technologies varies depending on weather conditions. There is a need for new processes that can store energy cheaply and reliably for months at a time, researchers say.
Engineers and geoscientists from the Universities of Edinburgh and Strathclyde used mathematical models to assess the potential of the process, called compressed air energy storage (CAES).
The team then predicted the UK's storage capacity by combining these estimates with a database of geological formations in the North Sea.
Porous rocks beneath UK waters could store about one and a half times the UK's typical electricity demand for January and February, they found.
Compressed air energy storage would work by using electricity from renewables to power a motor that generates compressed air. This air would be stored at high pressure in the pores found in sandstone, using a deep well drilled into the rock. During times of energy shortage, the pressurised air would be released from the well, powering a turbine to generate electricity that is fed into the grid.
A similar process storing air in deep salt caverns has been used at sites in Germany and the US.
Locating wells close to sources of renewable energy—such as offshore wind turbines—would make the process more efficient, cheaper and reduce the amount of undersea cables required, the team says.
The study is published in the journal Nature Energy.
Dr. Julien Mouli-Castillo, of the University of Edinburgh's School of GeoSciences, who led the study, said: "This method could make it possible to store renewable energy produced in the summer for those chilly winter nights. It can provide a viable, though expensive, option to ensure the UK's renewable electricity supply is resilient between seasons. More research could help to refine the process and bring costs down."
...…
And then amazingly an article about atmospheric separations for Mars dated from 2004. Some of the text is a bit garbled I think, and I wonder why they don't mention Argon, but it is an interesting proposal.
https://www.lpi.usra.edu/meetings/round … f/6036.pdf
Quote:
Space Resources Roundtable VI (2004) 6036.pdf
EXTRACTION OF OXYGEN FROM THE MARTIAN ATMOSPHERE C. England 1Jet Propulsion Laboratory, Pasadena, CA 91109, cengland@jpl.nasa.gov
A mechanical process was designed for direct extraction of molecular oxygen from the martian atmosphere based on liquefaction of the majority component, CO2, followed by separation of the lower-boiling components. The atmospheric gases are compressed from about 0.007 bar to 13 bar and then cooled to liquefy most of the CO2. The uncondensed gases are further compressed to 30 bar or more, and then cooled again to recover water as ice and to remove much of the remaining CO2. The final gaseous products consisting mostly of nitrogen, oxygen, and carbon monoxide are liquefied and purified by cryogenic distillation. The liquefied CO2 is expanded back to the low-pressure atmosphere with the addition of heat to recover a majority of the compression energy and to produce the needed mechanical work. Energy for the process is needed primarily as heat to drive the CO2-based expansion power system. When properly configured, the extraction process can be a net producer of electricity.
The conceptual design, termed “MARRS” for Mars Atmosphere Resource Recovery System, was based on the NASA/JSC Mars Reference Mission (MRM) requirement for oxygen. This mission requires both liquid oxygen for propellant, and gaseous oxygen as a component of air for the mission crew. With single redundancy both for propellant and crew air, the oxygen requirement for the MRM is estimated at 5.8 kg/hr. The process thermal power needed is about 120 kW, which can be provided at 300-500°C. A lower-cost nuclear reactor made largely of stainless steel could serve as the heat source.
The chief development needed for MARRS is an efficient atmospheric compression technology, all other steps being derived from conventional chemical engineering separations. The conceptual design describes an exceptionally low-mass compression system that can be made from ultra-lightweight and deployable structures. This system adapts to the rapidly changing martian environment to supply the atmospheric resource to MARRS at constant conditions.
The large amounts of liquid CO2 by-product that are produced enable a comprehensive martian surface architecture using this liquid as an open cycle working fluid. While most of the 1000 kg/kg oxygen is expanded for power recovery, a small fraction is stored and made available for emergency or backup power, transportation, and surface operations such as drilling. The availability of highly redundant backup power and transportation systems makes the MARRS concept particularly attractive for piloted missions to Mars.
The current study outlines an inherently flexible surface architecture for Mars exploration that uses nuclear heat, a compression-dominated process for extraction of atmospheric resources, and provides a mechanism for highly redundant and reliable operations. The amounts of minor components in the atmosphere, however, are uncertain. While the conceptual design for MARRS is based on a 0.13% oxygen concentration, the actual average value is now believed to be about 0.3%. Such a high value would allow even greater flexibility in design, and greatly reduce the energy and mass requirements to produce oxygen for the MRM. A more detailed design is needed to account for the uniquely high variability in composition, pressure and temperature that characterize the martian atmospheric environment.
……
Items this connects to:
Louis stared this one in 2008, it ponders pressurized air energy storage, and Methane production.
http://newmars.com/forums/viewtopic.php?id=6014
I would say that if you read the above PDF, if the processes can be done, then Methane can be produced by using microbes that can digest CO and H20. And as you might see from the readings above, compressed energy storage can be accomplished by combining porous rock and salt dome spaces with the above mentioned processes (And Heliostats, and solar panels).
BenVA has provided this recently, and I feel that it can be implemented in association with what if mentioned above.
http://newmars.com/forums/viewtopic.php?id=8887
While he mentions nuclear energy to warm the subsurface, I will also mention that Heliostats could be used also. First I suppose a source of electrical energy will liquify a portion of the CO2 in very compressed Martian atmosphere. Then that Liquid CO2 can be heated to a gas with Heliostats, and pushed into porous rocks like sandstone, or into salt domes if they exist. To extract energy, the CO2 can be vented anytime. Preferentially though it would be vented during the day, so that heliostats can heat it again on the way out.
VOID provided this:
http://newmars.com/forums/viewtopic.php?id=7552
Heliostats. Enough said.
VOID provided this:
http://newmars.com/forums/viewtopic.php?id=7150
Atmospheric Separations.
And I am sure there are other connections to this collection.
For instance there has been recent activity by SpaceNut on this thread:
http://newmars.com/forums/viewtopic.php?id=7327
Crops Aquatic. We don't need to get specific about how we get photons to SpaceNuts seaweed. Maybe a sort of natural greenhouse, maybe artificial light, maybe something else. But if you have a supply of CO and O2 in a ratio of 1 to 2 in abundance, and if you also have perchlorates, then you have plenty of Oxidizers to consume the CO to produce biomass and Methane, and also plenty of Oxidizers to provide breathing Oxygen, propellant Oxygen, and chemosynthesis using Oxygen and perhaps Perchlorates.
……
And then of course I always prefer reservoirs of water where possible, and I think they are very possible on Mars.
So, I think abundance in full, on Mars is a potential.
Done
Last edited by Void (2019-01-21 12:54:13)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#2 2019-01-21 19:39:48
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Atmospheric Resources Methods of Life Support.
I like the idea of having methane clathrates stored on the surface, maybe under regolith, in shaded areas where you won't get direct sun. This would potentially avoid the need for resource-expensive pressure chambers. When your methane electricity generator needs methane, your digger digs up some clathrate and puts them in the hopper head into the air lock. ![]() Not sure if you can do the same with oxygen - can they form clathrates?
Not sure if you can do the same with oxygen - can they form clathrates?
I believe that I have stumbled upon a set of references that if taken together point to a potentially good method for Mars.
This topic will also intersect several others such as "Atmospheric Separations" and others.Post # 1 content.
So, I think abundance in full, on Mars is a potential.
Done
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#3 2019-01-21 20:03:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,730
Re: Atmospheric Resources Methods of Life Support.
Louis, Methane hydrates, which form at low temperature and high pressure, are found in sea-floor sediments and the arctic permafrost.
https://en.wikipedia.org/wiki/Methane_clathrate
Methane clathrate (CH4·5.75H2O) or (4CH4·23H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice.
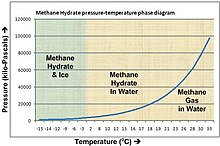
Methane Hydrates and Contemporary Climate Change
Methane hydrate is an ice-like substance formed when CH4 and water combine at low temperature (up to ~25ºC) and moderate pressure (greater than 3-5 MPa, which corresponds to combined water and sediment depths of 300 to 500 m).
1 MPa = 10 bar
1 Bar = 14.7 Psi
Mars air pressure
The atmospheric pressure on the Martian surface averages 600 pascals (0.087 psi; 6.0 mbar), about 0.6% of Earth's mean sea level pressure of 101.3 kilopascals (14.69 psi; 1.013 bar)
0.006 bar for mars surface pressure
Offline
Like button can go here
#4 2019-01-21 21:05:26
- Void
- Member
- Registered: 2011-12-29
- Posts: 8,923
Re: Atmospheric Resources Methods of Life Support.
I sort of buy into that SpaceNut, but I think it must be remembered that Martian permafrost is at a whole next level of cold I think relative to polar permafrost, and Antarctic permafrost, well maybe within reach. But I really don't care about creating Methane Clathrate.
I think that if you could store pressurized heated CO2 in porous rock, or salt domes (If they exist in a fashion usable), then it should be possible to store Methane in similar settings elsewhere. Clathrate will not be required, and could be an impediment to re-extraction.
But I also say if you review the materials of my first post you will see, that it seems worth exploring if there is a virtually infinite supply of CO and O2, in a proportion of 1 to 2 in quantity. CO is a relatively poor fuel, but would be a byproduct of a process where you store pressurized hot CO2 in porous rocks (Sandstone?), and/or salt domes.
And if you read the materials you would see that they expect to extract water from the atmosphere in this process, at the second stage of processing. So, if successful to that extent, you would have many of the main resources you would need.
And if you should need Methane, you might make it on demand by reacting the CO either with water biologically or by reacting it with Hydrogen you might extract from water. That second method could be completed biotically or abiotically (Per a Sabatier Reactor).
Whereas then process described for atmospheric separations was cryogenic, I also think that there may be room for improvements at some stages using membrane separations. And I do believe that Argon could really be an asset per SEP propulsion from Mars to Earth, and perhaps even from Earth to Mars (Even though Argon may be gotten more locally in the Earth/Moon subsystem.
I am fairly confident that pressurized temperature modified gasses can be stored in porous rocks on Mars, especially if icy permafrost exists as a near surface layer blocking leakage. I am very confident that salt domes if they exist will be useful for the same.
However in the case where it is possible to create a layer of water over porous rock that will perhaps work as well as what is proposed for the North Sea, for storing energy as a pressurized gas in porous rocks.
More about salt domes and natural gas:
https://www.power-eng.com/articles/2001 … orage.html
My guess is that if there were seas and lakes that did not drain to a "Next" body of water, then there should have developed salt domes in the lower basins of Mars.
What lies under the surface of the northern plains is relatively a mystery. However we do know that there are slabs of ice in places, and over those usually a dust/regolith layer perhaps 3-30 feet thick.
Some speculation even includes the possibility that some of the actual remnant of the ancient sea ice may be deeply buried. Not saying it is so, just saying that we don't have all the information we should want, and will need to gather.
But in actuality I think that if there are salt domes, there will be large quantities of natural gas to drill for, and of course natural gas has a large content of Methane. We do have seasonal appearances of Methane in the Martian atmosphere as it is. So something like natural gas occurs naturally on Mars. It may have a hard time emerging through the permafrost, especially during the winters.
Done
Last edited by Void (2019-01-21 21:18:12)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#5 2019-01-21 21:45:41
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,730
Re: Atmospheric Resources Methods of Life Support.
The electrolysis of carbon dioxide gives formate or carbon monoxide. https://dioxidematerials.com/technology … ctrolysis/
CARBON DIOXIDE REDUCTION AND WATER ELECTROLYSIS …
https://apps.dtic.mil/dtic/tr/fulltext/u2/673903.pdf
Electrochemical Conversion of Carbon Dioxide to Hydrocarbon Fuels
https://www.ems.psu.edu/~elsworth/cours … rochem.pdf
CO production from CO2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide
https://www.sciencedirect.com/science/a … 2016300439
Carbon monoxide production from carbon dioxide via isothermal reverse water–gas shift chemical looping (RWGS-CL) is studied with a modified iron oxide oxygen carrier material (80 wt% Fe2O3–Ce0.5Zr0.5O2). The material is characterized by TEM, XRD and thermogravimetry at temperatures from 750 °C to 850 °C and gas mole fractions of H2 and CO2 from 0.05 to 0.75, respectively. High temperature and high reactant concentrations favor the oxidation and reduction of the material during repeated redox cycles.
Of course we are still trying to fit what works within the mass envelope of flights to mars and being able to build on what does....
Offline
Like button can go here
#6 2019-01-22 14:33:11
- Void
- Member
- Registered: 2011-12-29
- Posts: 8,923
Re: Atmospheric Resources Methods of Life Support.
The production of CO, and so also Oxygen from CO2 could be important and appropriate depending on capabilities and needs.
I am probably more like the lazy man a boss will put on a job, to find out how to do it more effectively/efficiently. Maybe get more gain for the effort given. Or at least at times I have that level of conceit. Perhaps I will discover an easier way, maybe not, but I am likely to try.
Human desire to expend great efforts to achieve the ability to be leisure is probably a partial answer to how we now have the technology and organization of society that we have. We consider it to be productive of what we want. (With some bad side effects mounting that we need to solve for).
The second article I linked to in my post #1, describes a proposal to do atmospheric separations on Mars to achieve a greater chemical prosperity for humans of Mars, and even suggests that this could be done with an energy gain. I like that. Win Win? Well that would be the hope.
Here it is again:
https://www.lpi.usra.edu/meetings/round … f/6036.pdf
Quote:
EXTRACTION OF OXYGEN FROM THE MARTIAN ATMOSPHERE C. England 1Jet Propulsion Laboratory, Pasadena, CA 91109, cengland@jpl.nasa.gov
A mechanical process was designed for direct extraction of molecular oxygen from the martian atmosphere based on liquefaction of the majority component, CO2, followed by separation of the lower-boiling components. The atmospheric gases are compressed from about 0.007 bar to 13 bar and then cooled to liquefy most of the CO2. The uncondensed gases are further compressed to 30 bar or more, and then cooled again to recover water as ice and to remove much of the remaining CO2. The final gaseous products consisting mostly of nitrogen, oxygen, and carbon monoxide are liquefied and purified by cryogenic distillation. The liquefied CO2 is expanded back to the low-pressure atmosphere with the addition of heat to recover a majority of the compression energy and to produce the needed mechanical work. Energy for the process is needed primarily as heat to drive the CO2-based expansion power system. When properly configured, the extraction process can be a net producer of electricity.
So, I want to play with their method, hope to get more gain with less effort.
From Atmospheric Separations post #42, I have this:
Quote:
For Mars itself, I am interested in all of the gasses.
https://en.wikipedia.org/wiki/Atmosphere_of_Mars
Quote: (A little jumbled up)
2005
General information[1]
Chemical species
Mole fraction
Composition[1]
Carbon dioxide
95.97%
Argon
1.93%
Nitrogen
1.89%
Oxygen
0.146%
Carbon monoxide
0.0557%
You can correct me on my rough math. Excessive precision here is likely not warranted.
With the above numbers, if you could eliminate the CO2, and be left with everything else, I estimate that that multiplies the amount of each by ~25 times.
Argon
48.25%
Nitrogen
47.25%
Oxygen
3.65%
Carbon monoxide
1.3925%
I will see what that adds up to. 100.5425, which is close enough for estimations.
If that is a rough indication, I would say that you could already inject the mixture into a microbe farm, and get them to process the chemicals. Most likely that would eliminate the CO, as their food, and some of the Oxygen for them to breath, unless you made them breath perchlorates. They might be GM so that they could fix Nitrogen into fertilizer, or to produce hydrocarbon liquids or Methane.
So depending on the breathing process the microbes used, you could end up with:
Argon
>48.25%
Nitrogen
>47.25%
Oxygen
~2.2575 to 3.65% (Depending on what the microbes use as oxidizer).
0.0% Carbon monoxide
Looking at this quote again:
Quote:
A mechanical process was designed for direct extraction of molecular oxygen from the martian atmosphere based on liquefaction of the majority component, CO2, followed by separation of the lower-boiling components. The atmospheric gases are compressed from about 0.007 bar to 13 bar and then cooled to liquefy most of the CO2. The uncondensed gases are further compressed to 30 bar or more, and then cooled again to recover water as ice and to remove much of the remaining CO2. The final gaseous products consisting mostly of nitrogen, oxygen, and carbon monoxide are liquefied and purified by cryogenic distillation. The liquefied CO2 is expanded back to the low-pressure atmosphere with the addition of heat to recover a majority of the compression energy and to produce the needed mechanical work. Energy for the process is needed primarily as heat to drive the CO2-based expansion power system. When properly configured, the extraction process can be a net producer of electricity.
It seems to me that their first cooling and pressurizing processing divides the Martian atmosphere into two initial mixes. 1) Relatively pure Liquid CO2, and 2) A remainder of uncondensed gases, which will be composed of a relatively reduced amount of CO2, Water Vapor, and nitrogen, oxygen, and carbon monoxide. I will also add that disturbingly they seem to have neglected Argon, which I would expect to be present.
I might wish to replace the cryogenic step #1 with something else, if that something else worked better. That is if it required less effort and produced better results. But I will for this discussion, stick with their process.
So, for #1 they have a quantity of liquid CO2. This suggests that during the day a collection of heliostats or a nuclear process could be used to heat it to a high pressure vapor, and it could be directed in two different ways. a) Some could be vented through a turbine to atmosphere ambient, to produce electrical energy. b) Some could be pumped into underground reservoirs such as porous rocks and or salt domes. This would store pressurized CO2 and also Heat. So you might get an energy gain, and yet energy storage as well, and a byproduct of; "2) A remainder of uncondensed gases, which will be composed of a relatively reduced amount of CO2, Water Vapor, and nitrogen, oxygen, and carbon monoxide. I will also add that disturbingly they seem to have neglected Argon, which I would expect to be present.".
We could continue on with their process as they stated, but I have my own ideas for #2.
I would put it into a microbe farm. Perhaps in a ice covered reservoir. Perhaps in a tunnel dug boring company style. Or if you like a fiberglass container. Or, whatever.
If we do that alone, we can first hope that their is not so much CO2 that it is poisonous for the microbes, and does not make the water too acid.
If that is all we do, then I expect a result of;
CO2 Unknown amount....
?%
Argon
>48.25%
Nitrogen
>47.25%
Oxygen
~2.2575%
0.0% Carbon monoxide
So, although we could have tried to separate out the CO and the Oxygen, here I have opted to simply exchange the loss of all of the CO and some of the Oxygen, to the microbes, but to then obtain a less poisonous mixture, and also biomass which can be converted into better fuels such as Methane, and which might also be used to grow mushrooms as well. Not all of the Oxygen is depleted.
So then you have a simplified mix, and could use some method to separate out mixes of the components. Perhaps even beneficiating the Oxygen content of one sub-mixture to the point of turning it into breathable air. Say an Argon/Nitrogen/Oxygen mix.
But there are other chemicals you could add to the mix. They have a toxic potential, but certain microbes may handle them OK.
-Perchlorates.
-Dune Materials and basalt soils not yet fully Oxidized. (This will present the danger of Hexavalent Chromium as SpaceNut has alerted me previously).
The reason to add the Perchlorates is some microbes may be able to use it for an Oxidizer I presume.
The reason for the basalt sand dune materials its that I expect a corrosion process to generate Nitrous Oxide and Hydrogen.
I am not sure about the Nitrous Oxide. The microbes may utilize it. Anyway the Hydrogen produced should serve as a getter for the CO2 and also for CO. Microbes expected to utilize all of them.
So, the hope would be to eliminate the CO2 by consumption by microbes which would also consume Hydrogen.
If some microbes use Perchlorates, then some of the CO will be consumed by them without using up dissolved Oxygen.
In that case, then I would hope for an approximate result such as this:
CO2
0%
Argon
>48.25%
Nitrogen
>47.25%
Oxygen
>~2.2575%, maybe as much as 3.65% (Depending on what the microbes use as oxidizer).
0.0% Carbon monoxideAnd possibly Methane, and surely some biomass. (Which can be converted to fuels such as Methane, fish food, mushroom food, or even human food).
And then if you have excess electricity, you may split CO2 or H20 and add those chemicals to the batch as well if that will give you a useful gain.
Done.
Last edited by Void (2019-01-23 11:31:08)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#7 2019-01-22 21:01:43
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,730
Re: Atmospheric Resources Methods of Life Support.
Co2 liquification for mars...
https://science.nasa.gov/science-news/s … iticalco2/

The critical point, denoted by the big gray dot, is a special combination of temperature (Tc=31 C) and pressure (Pc=73 atm) where CO2 has properties of both liquid and gas.
We know that the night temperatures will cause frost of co2 on a cold plate from super cooling it but we need lots more than frost.
Frozen carbon dioxide, often called dry ice, doesn’t melt. At atmospheric pressure it sublimes at −78.5 °C going straight from solid to gas.
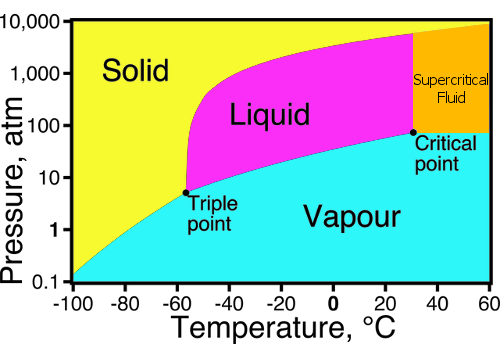
At 0.006 Bar for a 1 meter cube its not much when we need to multiply the boxes by a 835 times to get to a 5 bar at a temperature of -55C for a 1 meter of liquid co2...
Offline
Like button can go here
#8 2023-05-20 16:42:32
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,730
Re: Atmospheric Resources Methods of Life Support.
More pressure and heat to process and then to cool the end product to get gas separation to occur. Mars is not going to be low energy....
Offline
Like button can go here
#9 2023-06-07 11:37:04
- Void
- Member
- Registered: 2011-12-29
- Posts: 8,923
Re: Atmospheric Resources Methods of Life Support.
Just wanted a less intrusive space to paste this:
https://www.msn.com/en-us/news/technolo … r-AA1ceHR7
Quote:
PEC devices use semiconductor materials to convert solar energy directly to chemical energy to produce hydrogen and oxygen without requiring the intermediate production of electricity. The technology is the subject of intense research on Earth because it might help in the sustainable energy problem, but its potential in space has yet to be studied.
Done
Last edited by Void (2023-06-07 11:39:51)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here