New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#51 2018-11-19 00:20:09
Re: Steam powered rovers
I mean, mining dry ice at the pole to use later is an idea but I don't think it's worth doing from an energy perspective unless your settlement is physically located at the poles.
I don't see why you would want to capture the CO2 once you've used it: The low temperature, low-pressure gas output of the system is not really any more valuable than the gas in the atmosphere. Plus, the volume will be huge: A 50 kW system operating overnight will generate 1.2 million cubic meters of ambient-pressure gas, enough to fill a cube 100 meters on a side.
-Josh
Offline
Like button can go here
#52 2018-11-19 07:43:04
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,889
Re: Steam powered rovers
I mean, mining dry ice at the pole to use later is an idea but I don't think it's worth doing from an energy perspective unless your settlement is physically located at the poles.
Thanks to SpaceNut for the chart and report on the natural dry ice cycle at the poles. Thanks to JoshNH4H for confirmation the use of dry ice from the poles is feasible from an engineering point of view.
I'd like to approach this from the business opportunity perspective. The value of a product is ultimately determined by human beings, and in the capitalist system, that value is usually evaluated in competition with other alternatives.
The work done by students Chase Bishop and James Thompson (reported in post #3 Life support systems >> Dry ice pneumatic tool >> 2018-11-08 15;41:05) appears (to me) to demonstrate that a harvest of dry ice by an entrepreneur would have economic value in an economy where customers have equipment capable of using it. At a minimum I would anticipate the customers would have solar energy collectors/concentrators to apply to the dry ice, and mechanical devices to take advantage of sublimed CO2.
The cost of transport would be a major factor in determining the competitive value of mined dry ice. In human history on Earth, railroads on land have often yielded competitive results which have enabled products like coal to reach markets far from the mines. Pipe lines do not seem appropriate for this application, but the interesting scientific experiments reported elsewhere in the Forum, showing skittering of dry ice over a raised ridged roadway might be worth considering. In that scenario, a block of ice might skitter itself from the pole to the equator and still have some of itself left over for customer use.
Without anything (at this point) to work with for data, I can imagine a solution that brings carloads of dry ice from the poles to the equator, or mid-latitudes, at a cost which is competitive with costs of alternative energy delivery systems which may be offered.
(th)
Offline
Like button can go here
#53 2018-11-19 10:22:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
As always we have a couple of applications an uses being discussed.
As a stationary power plant the exhaust co2 is clean where as the collected frost will contain dust which would clog filters over time.
As a small mobile vehicle we would be looking to exhaust the co2 or collect it at exiting pressure somewhere less than the pressure used to create work and compress that into a high pressure tank for direct injection.
Later in years we would mine plus transport over a variety of means and probably when a nuclear plant is at the poles transport it by pipeline to where we would want it.
Offline
Like button can go here
#54 2018-11-19 10:25:40
Re: Steam powered rovers
Hey tahanson,
It's certainly conceivable that mining dry ice at the poles could be economical, but it's hard (for me) to imagine that it would actually make sense unless the end user is actually at the poles.
You used coal as an analogy, and it's a pretty decent one. However, rather than showing the potential value of CO2 mining I believe it shows why it probably won't be worthwhile.
I believe the appropriate comparison is the system we were discussing before: a refrigeration system that uses energy to freeze CO2 out of the atmosphere. Here are the costs and benefits of such a system:
Costs:
Requires industrial equipment
Consumes energy
System has not been designed fully yet
Benefits:
Generates ~1 kg per kWh of inert gas (primarily Nitrogen and Argon)
Works equally well anywhere on Mars
Now, by comparison, here are the costs and benefits of pole-mining:
Costs:
Requires an ongoing supply of human labor (All mining does)
Consumes energy
Requires shipment over thousands of kilometers
Requires industrial equipment
System for mining a substance that sublimes at 195 K has never been built and would require some tweaks to existing techniques
Benefits:
Will produce water as a byproduct
In my view, the question is: Is more labor and thousands of km of shipping worth saving yourself 150 kJ/kg? In my opinion, it is not. It's good to compare this to coal: Coal on Earth has an energy content of 30 MJ/kg, 200 times greater than the energy you save. Because the energy content is so high, it really is worth pulling out out of the ground and shipping it to the point of use (or at least it was in times past). I question whether mining CO2 from the poles would save any energy at all.
-Josh
Offline
Like button can go here
#55 2018-11-19 11:28:17
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Steam powered rovers
Would the system exhaust to ambient pressure, or are there benefits from using a (very large...) tank to hold it at say 100mb? What's the efficiency penalty of going from 10 bars to 1 bar, rather than 10 bar to ambient? It would certainly be easier to repressurise it, no?
Use what is abundant and build to last
Offline
Like button can go here
#56 2018-11-19 12:28:17
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,889
Re: Steam powered rovers
Hey tahanson,
It's certainly conceivable that mining dry ice at the poles could be economical, but it's hard (for me) to imagine that it would actually make sense unless the end user is actually at the poles.
Benefits:
Will produce water as a byproduct
In my view, the question is: Is more labor and thousands of km of shipping worth saving yourself 150 kJ/kg? In my opinion, it is not. It's good to compare this to coal: Coal on Earth has an energy content of 30 MJ/kg, 200 times greater than the energy you save. Because the energy content is so high, it really is worth pulling out out of the ground and shipping it to the point of use (or at least it was in times past). I question whether mining CO2 from the poles would save any energy at all.
For JoshNH4H ... thank you for considering the suggestion of dry ice mining for a commercial competitive enterprise.
A successful business is not necessarily the one that spends the least for raw material. I would like to see robust competition between the pole miners and the make-your-own-dry-ice community. However, I was surprised to see provision of water as a benefit. While there is plenty of water ice at the poles, (I gather), my business plan did not include harvesting any of it.
Can you point me to a post or a link that explains how water ice might occur when dry ice sublimes?
It might make sense to harvest water ice while collecting dry ice. That is a different question.
(th)
Offline
Like button can go here
#57 2018-11-19 14:00:37
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
Tele robotic machines will be used for a polar mining operations where the only limit will be the power constraint that we have to make use of.
Polar mining operations will yield dirty dry ice to begin with so the question is dry ice that contains dust is that anymore energy intensive for processing as that is the second tier of refinement for use?
We do know that mining will be more energy intense than a free air condensation frost process of collection depending on how much energy must be used to make it freeze out of the air and for scraping it off the plates as the first tier energy consumption.
Offline
Like button can go here
#58 2018-11-19 14:18:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,889
Re: Steam powered rovers
For SpaceNut in Post #57 ...
Thanks for support of teleoperation as a viable option for commercial dry ice (and possibly water) mining at Mars.
The pdf at the following link is dated 1997, but it shows that even then, teleoperation of heavy mining equipment was moving forward in Australia.
http://acs.ist.psu.edu/misc/dirk-files/ … rfaces.pdf
I would expect very limited human on site presence for any mining activity on Mars.
(th)
Offline
Like button can go here
#59 2018-11-19 14:35:53
Re: Steam powered rovers
Would the system exhaust to ambient pressure, or are there benefits from using a (very large...) tank to hold it at say 100mb? What's the efficiency penalty of going from 10 bars to 1 bar, rather than 10 bar to ambient? It would certainly be easier to repressurise it, no?
I've been assuming the system exhaust would be at ~100 mbar and 195 K and released to ambient.
The exhaust pressure determines the number of reheat cycles you can do, which strongly influences the efficiency of the system. So, for example, here's the system EROI (Work out divided by work in) and isentropic efficiency and exhaust pressure as a function of the number of heating cycles. Note that the pressure declines by 62% after each heating cycle. I assumed an initial pressure of 10 bar.

Negative efficiency values correspond to an EROI less than one. All chemical batteries have negative efficiencies when measured in this way (although it's not clear that you could calculate an actual numerical value for the efficiency because the denominator, heat input, is zero). All of these values will be lower in a real system: Inefficiencies will make the work input higher and the work output lower. Furthermore, friction between stages will cause pressure loss.
For an input pressure of 10 atm, I would use 5 heating cycles to release gas at 80 mbar. This means you can get roughly twice as much energy out of each kg of CO2 as if you release at 1000 mbar.
If we were using compressor pumps it would absolutely be the case that pressurizing from 7 mbar to 1000 mbar would be the most energy intensive portion. Work is path dependent, which means that when you go straight from 7 mbar to 10,000 mbar in a small, constant volume (as when you're melting dry ice) you don't do a lot of it.
There's also the consideration of storage: Gases at one bar really are not dense. Our hypothetical 50 kW overnight power system would require 27 tonnes of LCO2, which at 1 bar and 200 K will take up about 11,000 m^3. Using the same assumptions as for the 10 bar LCO2 tank (post #17) this tank will mass roughly 270 tonnes and have a diameter of 30 meters.
Last edited by JoshNH4H (2018-11-19 15:10:44)
-Josh
Offline
Like button can go here
#60 2018-11-19 14:49:35
Re: Steam powered rovers
While there is plenty of water ice at the poles, (I gather), my business plan did not include harvesting any of it.
Can you point me to a post or a link that explains how water ice might occur when dry ice sublimes?
It might make sense to harvest water ice while collecting dry ice. That is a different question
No substance in nature is pure. There is dry ice at the poles and there is water ice at the poles. Any attempt to mine the one will therefore also produce the other. Admittedly, I do not know what the ratio of the one to the other will be, but I'm fairly confident that whatever you mine will be less than 100% pure. Water admixed into the dry ice presents both potential benefits and potential challenges.
Only the real world will tell, but competition between different technologies only works when the two technologies are close enough to parity that they can both be profitable at the same time. For example, it's possible to get from New York to Los Angeles on foot, on bike, by plane, by train, and by car. People do the latter three to varying degrees, but only do the former extremely rarely because it's so much harder. Nobody will open a business to bring cargo to LA by bike because it couldn't possibly be profitable.
It's important to mention that mining by teleoperation is not a labor-free activity. In general the use of robots requires substantial setup and oversight. Just because the workers aren't out on the cap with a pickaxe doesn't mean their labor doesn't count.
-Josh
Offline
Like button can go here
#61 2018-11-19 20:14:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
Here is an example of a cold plates construction

Here is the format of the chamber with the plates installed

Close the door before dawn and turn the active cooling off and wait for the thermal heating from the sun to happen.
As it phase changes to gas pump it to the storage tank for future use.
The other process would be to close the door before dawn and scrape the plate clean into a holding chamber for direct use in a thermal bottle.
Offline
Like button can go here
#62 2018-11-20 15:05:58
Re: Steam powered rovers
Hey SpaceNut,
That's an interesting device that would work for some applications but probably isn't adequate for this one. The problem here is volume. A 50 kW overnight system is going to need something in the range of 15-20 tonnes per day of LCO2. A batch process in which you need to manually scrape frost off of a plate just doesn't have the capacity or the efficiency that would be needed to make this workable.
There are two related technical issues that I see with the process of freezing CO2 out of the atmosphere.
The first is the low density, and therefore low rate of heat transfer, of the Martian atmosphere.
The second is that CO2 frost on solid surfaces will be hard to recapture for use and will reduce the rate of heat transfer from the refrigeration unit to the atmosphere. This is a problem because the CO2 will tend to frost on precisely the surfaces being used to freeze it.
I believe I may have a solution which addresses both problems: Use the ambient CO2 atmosphere as your working fluid.
I mentioned refrigeration very briefly in post #26. I would like to go into somewhat more detail here. Refrigerators (or heat pumps, which is the more general term) are thermodynamic engines very similar to the power generating cycles we've been discussing in this thread. However, instead of generating power while transferring heat from hot to cold, they transfer heat from cold to hot while consuming power. Here's how a refrigeration cycle works, idealized and in the abstract. Note that the "fluid" in this case usually means a gas:
Isentropic compression: In this stage, the working fluid is compressed with no heat transfer. Compression causes the fluid to heat up to above the ambient temperature.
Heat Rejection: In this stage, the working fluid is cooled by releasing heat to the environment
Isentropic Expansion: In this stage, the working fluid expands through a piston or turbine and cools, returning some (but not all) of the work done in stage 1. At the end of this stage, the fluid is cooler than it was originally.
Heat Absorption: In this stage, the working fluid absorbs heat from the cold side. At the end of this stage the fluid is at the same temperature and pressure as it was at the beginning of stage 1. The cycle then repeats.
In general, the system works best when the "cold" temperature corresponds to the condensation of the working fluid at the operating pressure of the system. The reason for this is that this creates a uniform temperature on the cold side and increases the density of the fluid. All else being equal, both of these improve heat transfer.
This drives the choice of fluid for the system. Historically, some of the first refrigerators used Ammonia. Later, new gases were developed: Chlorofluorocarbons (CFCs). These were banned for environmental reasons and replaced with fluorocarbons (FCs) and hydrofluorocarbons (HFCs). These gases also raise some environmental concerns and lately there has been a movement to consider some other gases.
One refrigerant gas which saw some use in the early years and sees increasing use today is Carbon Dioxide (which is known as R744 when it's being used in a refrigerator/heat pump). This makes it sorta convenient for our use: We can, in some senses, use Carbon Dioxide to refrigerate itself.
Here's how I propose to do that, in the abstract:
Start with ambient air and filter out the fines to the extent that it's possible to do so. An electrostatic system seems like the best way to get the really fine particles out to me. This air isn't intended for human consumption so it's a question of machine wear vs. filtering cost.
Compress the air using a turbine. In this case the pressurization we're looking at is pretty mild: As an example, assuming you want to pressurize from 230 K to 270 K (and assuming CO2 behaves like an ideal gas) the pressure differential will be by a factor of two. In the case of Martian ambient, that means you're pressurizing from 0.7 kPa to 1.4 kPa.
Cool the gas back down to ambient: Use a heat exchanger that radiates to the surrounding environment to cool the gas back down towards 230 K. Ammonia might be a good working fluid for this purpose because of its condensation temperature/pressure curve.
Expand the gas through a second turbine and allow it to cool. Ideally the parameters of this first stage would be such that the gas would cool to the sublimation temperature of 195 K
Compress the gas a second time and remove the heat a second time
Expand the gas through a turbine. In the first instance, the gas ended up at a lower temperature than when it entered. In this instance, the temperature can't fall because the gas already started at its sublimation temperature. Instead, some of the gas condenses out as dry ice. By cleverly designing the gas flow you can get this dry ice to be deposited at certain select points, where it can be moved batch by batch into the melting chambers.
No more than roughly 10% of the gas can condense out in any one cycle for the turbine to work correctly. Of the remaining gas, some is tapped off to be used as feedstock for N2 and Ar [this will be more energy intensive because the CO2 needs to be removed entirely but will also produce some dry ice along with useful quantities of Nitrogen and Argon buffer gases] or released to the atmosphere (this is necessary to prevent the mass of gas in the system from increasing forever as N2 and Ar build up) and most is redirected back to the input for the second stage, where it is mixed in an appropriate ratio with gas from the first stage.
My knowledge of refrigeration and chemical process (this system straddles the boundary) is not great, but I think this is the best way to meet our requirements.
-Josh
Offline
Like button can go here
#63 2018-11-20 16:11:46
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
The first group of 4 steps are for how air conditioning is done.
The next 7 step group is a hybrid system.
https://www.powermag.com/carbon-dioxide … -transfer/
Analysis of Super critical Carbon Dioxide Heat Exchangers in Cooling Process
http://www.sco2symposium.com/www2/sco2/ … hanger.pdf
102 pg Tutorial Heat Exchangers for Supercritical CO2 Power Cycle Applications
example product CO2 liquid separators with internal exchanger - CO2 Productions
http://ocscold.it/en/co2-productions/co … -exchanger

https://webbook.nist.gov/chemistry/fluid/
AIR COOLED HEAT EXCHANGERS FOR CO2 REFRIGERATION CYCLES
Since most of the dust will be metal particles the electrostatic will tend to short out quite easily so I would make use of a magnetic sieve and reverse air back wash to remove the particles once the magnet is off.
Offline
Like button can go here
#64 2018-11-20 16:43:13
Re: Steam powered rovers
Since most of the dust will be metal particles the electrostatic will tend to short out quite easily so I would make use of a magnetic sieve and reverse air back wash to remove the particles once the magnet is off.
metal particles
I'm sorry, what?
Planning to have a look at the rest of your references tonight
-Josh
Offline
Like button can go here
#65 2018-11-20 16:47:57
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
Carbon dioxide, or CO2, behaves in very unusual ways and allows you to see the three states of matter in one material. It freezes directly from a gas into a solid, called dry ice, at normal atmospheric pressures, bypassing the liquid phase entirely. This transformation happens at temperatures below -109.3 degrees Fahrenheit at a pressure of 1 atmosphere.
Problem 1 is we are not at 1 atm on mars so temperature is lower to make the state change on mars.
If you increase the pressure to 5.1 atmospheres, which is about 75 pounds per square inch, and maintain the temperature below minus 69 degrees Fahrenheit something very interesting happens. Known as the triple point, these conditions allow CO2 to coexist in the solid, liquid and gaseous states.
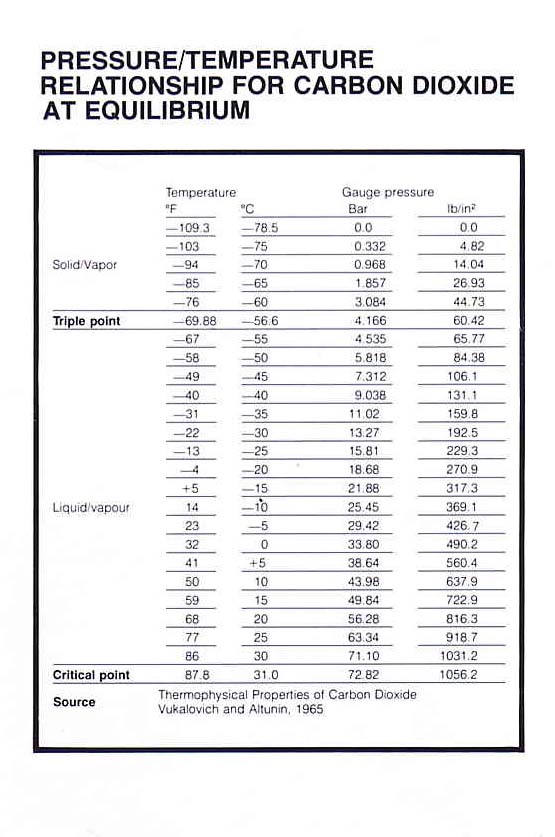
Offline
Like button can go here
#66 2018-11-20 21:48:22
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,432
Re: Steam powered rovers
I'm still reading about this, but I think a closed-loop SCO2 system is the way to go, even if that means a molten salt solar thermal power tower has to supply enough heat to both suck the CO2 out of the Martian atmosphere at near-vacuum pressure and compress it to LCO2 before the system is completely ready for use. To give everyone an idea about how large a 50kW SCO2 gas turbine is, the outer diameter of the blades is approximately the size of a US quarter and the shaft diameter is smaller than a US dime. The 10MW model weighs about 150lbs and its power density is about 89MW/m^3.
Here's a couple articles and papers / presentations about SCO2 systems:
Supercritical CO2: The Path to Less-Expensive, "Greener" Energy
Supercritical CO2 Eyed for High Power-Density In Electric Generation
Workshop on New Cross-cutting Technologies for Nuclear Power Plants
Commissioning of a 1 MWe Supercritical CO2 Test Loop
Supercritical CO2-Brayton Cycle - Potential benefits, applications, status and plans
Offline
Like button can go here
#67 2018-12-03 19:40:49
Re: Steam powered rovers
Hey all,
Sorry for my long absence in this thread. I was travelling over the holiday and unable to get the time together to have a look at things on the forum and reply.
Anyway, I've gone and had a look at some of the stuff you guys posted about sCO2 as a working fluid. I think it's a promising technology with a substantial range of applications that may or may not be optimally suited for Mars. In this post, I will go into more detail on why.
First, the good: The use of sCO2 in a thermodynamic engine has the potential to increase efficiency and decrease size at the same time. This is a rare case where a new development seems to be strictly better than what came before under all circumstances. And I want to be clear that under most circumstances, particularly on Earth, I really do think this is better. However, I don't think it's necessarily better under all circumstances. To explain why, I will start with two simple numbers: The pressure and temperature of CO2's critical point:
305 K (32 C and 89 F) and 7.4 MPa (74 atm). No portion of the thermodynamic cycle can operate below that temperature or below that pressure, else you lose the benefits of using a supercritical fluid.
These references all correctly note that denser fluids require less energy to compress, which is a big deal. The low-temperature engine I described above is not exactly typical, but I'm sure yo all noticed the fact that the low efficiency was due in large part to the work required to freeze CO2 out of the air.
I have three additional observations about this sort of system, one neutral and two drawbacks.
Neutrally, it seems that much of the efficiency gains come from a higher operating temperature. There is certainly nothing wrong in the abstract with operating at a higher temperature but it helps to make clear where the efficiency gains are really coming from.
Negatively, the pressures used in this system are likely to be enormous, far larger than comparable systems in use today and maybe even higher than a rocket engine like the SSME. If the low-pressure portion at the system is at 7.4 MPa, the high pressure portion will be at a substantially higher temperature. The Sandia report cited a maximum pressure of 14 MPa and the GE report mentioned operating pressures above 25 MPa. For reference even the SSME operates around 20 MPa.
Also negatively, such high pressures and temperatures operating in a small volume generate a huge challenge in designing a turbine to withstand them. Turbines already typically operate with advanced materials; this combination of high temperature, high pressure, and extreme force (large changes in pressure over short distances) are going to be really challenging to deal with, possibly at the very limits of materials as they currently exist. As a point of comparison, the surface of Venus is around 9 MPa and 450 C, which is to say it's actually a less hostile environment in many ways than the interior of such an engine.
I don't mean to claim that it's impossible to build such an engine, or even that it's excessively difficult. It's not. What I do want to point out is that the extreme operating conditions will produce an engine that uses advanced materials and requires thorough (read: expensive) design and testing to operate safely and reliably. It's precisely the kind of engine that you would consider for grid power on Earth, where efficiency is critical and system lifetimes are measured in decades.
This is what makes me question whether it's really the best choice for off-planet use. This scCO2 engine appears to approach a rocket engine in its conditions and complexity, and I'm sure we're all well-aware of the extent to which launches are a critical failure point. Efficiency, in general, takes a backseat to reliability for pioneering missions or settlements. This is particularly true of something as critical as an electrical generator. I am also curious if it would be possible to build a generator small enough to work in the range of our power needs without big losses in efficiency.
When looking at thermodynamic engines, as is often the case, there is no one correct answer but there are many wrong ones. I would say this falls within the group of reasonable options--the biggest concern to me being that it is not now nor has it in the past been in common use. Chicken and egg, I know.
-Josh
Offline
Like button can go here
#68 2018-12-03 22:00:54
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,432
Re: Steam powered rovers
Josh,
I did take note of the extreme temperatures and pressures, but that seems to be the price to be paid for low mass and high efficiency. Every aspect of the system requires superlative engineering and materials, but it's also hard to argue with how compact and efficient it is. Required material properties notwithstanding, from a mechanical complexity standpoint it's actually pretty simple in comparison to what NASA was working on in the 1980's.
I must also point out that SpaceX did the same thing to amp up the performance of Raptor. Their new engine is operating at extreme pressure, even though it's intended to be fully reusable. In decades past, I'd say that was a recipe for disaster. Test equipment and methods for measuring stress and fatigue life are so much better now that there's really no comparison between what's practical now and what was merely achievable just 20 years ago. Apart from that, most hydraulics used in mobile construction equipment operate at 35MPa or better and it's been that way since the 1990's.
Offline
Like button can go here
#69 2018-12-04 04:25:05
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Steam powered rovers
There are some difficulties associated with making practical machines for use with CO2. I don't say that it is not possible to make effective machines, just that these factors have to be considered.
For supercritical closed cycles (Brayton or Sterling) the equipment would actually be pretty small unless used in something like stationary generator applications. In very small machines efficiency tends to fall off due to manufacturing tolerances and operating clearances. I don't care how good your bearings are, they still need a clearance to the shaft. This means that very small compressors and turbines have much higher leakage rates, proportionately, than large machines doing the same function. You will not be able to get a close approach to the theoretical cycle efficiency with a very small machine
Small machines are also much more vulnerable to even a small degree of fouling, so more effort must be made to get the fluid perfectly clean. This is not such an issue with closed cycles, but is a major consideration for open cycles exhausting to atmosphere.
For an open cycle exhausting to Mars atmosphere the volume of the exhaust will be very large, because of the low ambient pressure. This, in contrast to the supercritical closed cycle case, means that the low pressure turbine will be very large, as will the exhaust ducting.
Anyone who has discharged a CO2 fire extinguishers will be aware of what happens when you expand CO2 from liquid to atmosphere. You get a lot of CO2 frost forming in the jet. This effect has to be avoided in any cycle to avoid deposition in the exhaust tract of the machine, so additional energy must be available to prevent this. The additional energy might be a bit of heating of the exhaust ducts and machine casing, for instance, but wherever it comes from the need for it will reduce the efficiency of the machine.
Offline
Like button can go here
#70 2018-12-04 08:46:51
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,432
Re: Steam powered rovers
Elderflower,
I agree about the point that small machines can and do have tolerance issues that are difficult to overcome, but the efficiencies posted were not theoretical in nature. The data comes from actually having built and operated small scale machines at the 50kWe to 10MWe output levels. That's in the documents I linked to. In point of fact, I also posted that I thought that the primary loop should be closed cycle. Minimization of issues associated with atmospheric contaminants, such as iron oxide, in the working fluid is just one of several reasons to use a closed primary loop.
Offline
Like button can go here
#71 2018-12-04 15:12:09
Re: Steam powered rovers
Assuming a reliable working model is built I think this would be a great technology to use in rocket applications and for launch from earth in general. 10 MW in 150 lb works out to roughly 150 kW/kg, an incredibly high number. The SAFE-400 reactor generated 400 kWt with a reactor mass of 540 kg. With a 40% efficient scCO2 cycle you could get 160 kWe from this. Scaling up to 10 MW, you would expect a reactor mass of 13.5 tonnes. Against this, 150 lb is a rounding error. No doubt major improvements are possible on the reactor side, especially at scale.
In space, there's also good reason to believe that a system of solar concentrating mirrors will be more reliable and have a lower mass. What I'm getting at is that this has the sort of power-to-weight ratio that makes short-duration, interplanetary electric propulsion missions begin to look doable (or at least possible), something which I have been deeply skeptical of for a long time.
On-planet, I agree that this would work well with a molten salt thermal battery/solar power tower setup for both daytime and nighttime power generation. Dust storms are another issue, for which a reserve of chemical energy is probably a better solution.
I think this is a good example of how earth-supply and local manufacture will have different technological results. For Earth-supply, mass is key and high technology/precise manufacturing is readily available and you'll almost certainly look to something like this. Local manufacture, on the other hand, will be somewhat rougher. Land on Mars is nearly free (the only cost really is opportunity cost) and a larger mirror array to compensate for a lower engine efficiency (but correspondingly cheaper manufacture) seems like a good trade to make.
-Josh
Offline
Like button can go here
#72 2018-12-04 18:41:30
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
The first problem faced by man is coming up with a working fluid from Mars atmospheric pressure on the Martian surface when it only averages 600 pascals (0.087 psi; 6.0 mbar), about 0.6% of Earth's mean sea level pressure of 101.3 kilopascals (14.69 psi; 1.013 bar).
Once we have the desire volume of pressure desired for any of the gaseous content that mars has; then we can build a system for processing it and of its energy required to do so.
Which leads to what can we do with solar as in concentrating and those working fluids in closed or open designs. The Stirling engine comes to mind.
https://www.grc.nasa.gov/www/tmsb/dynam … solar.html
digitalassets.lib.berkeley.edu/techreports/ucb/text/EECS-2007-172.pdf
https://www.springer.com/cda/content/do … 823-c2.pdf?
www.iieta.org/sites/default/files/Journals/IJHT/35.03_06.pdf
https://en.wikipedia.org/wiki/Solar_thermal_energy
We go through so many topics and here is a related to the atmosphere use.
Mars Atmospheric Kinetic Engine
http://www.travisdeyle.com/files/public … mPaper.pdf
Inflatable Membrane Solar Concentration Systems for Space-Based Applications
Offline
Like button can go here
#73 2018-12-05 18:37:50
Re: Steam powered rovers
SpaceNut-
I think the Mars Atmosphere Kinetic Engine is the one I had in mind originally.
kbd512-
I'm very excited by that 150 lb per 10 MW number but I've been having trouble finding it in any of the references. Can you point me to where you found it?
-Josh
Offline
Like button can go here
#74 2018-12-05 19:48:10
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,254
Re: Steam powered rovers
I was just looking at the rotary engine and how if we used continuous heat from an RTG that would allow near mars pressure air to be used directly and use the output side to work as a step up compressor.
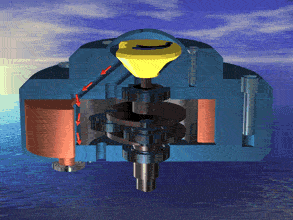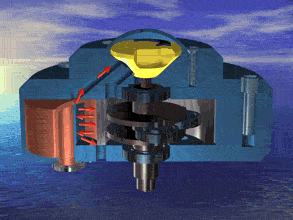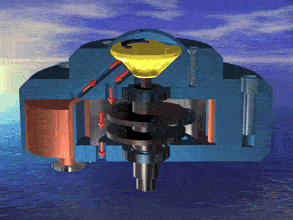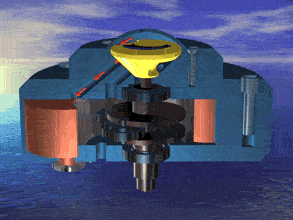
Inject the mars air into the chamber where its heated by the fluid jacket to make the pressure rise in the chamber to make it move.
So multiple chambers would increase the amount of movement.
http://runingonair.blogspot.com/2008/12 … y-air.html
Offline
Like button can go here
#75 2018-12-06 10:58:14
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,432
Re: Steam powered rovers
Josh,
Apparently it's just the turbine assembly itself, exclusive of the housing. There's no telling how much the housing weighs, but it won't be lightweight. I seem to call reading somewhere that as a general rule of thumb the turbine casing is about 3 times as heavy as the turbine. In any event, the rotating assembly is a single piece design that is 54" in length, 7" in major diameter, 4" shaft, and weighs 150lbs. The new industry buzzword for these types of sCO2 turbines "ultra supercritical" gas turbines. The steam-based RDK 8 system already running in Germany operates at up to 4,000psi. It's 47.5% efficient, which is better than most or maybe all other coal-fired power plants.
Here's the source from GE:
This Scientist's Got The Power (Plant) In His Hands
10 MW Supercritical CO2 Turbine Test - NREL
I believe that the video actually shows components from the 1MWe system that has already been built and tested:
I discovered from reading that the 10MWe pilot plant is due to begin operation in 2020, so it's still under construction at SwRI's headquarters in San Antonio, TX. SwRI and some other company partnered with GE. The 10MWe plant is not cheap, either. They received an $80M grant from DoE for the 10MWe proof of concept after the successful demonstration of the 1MWe unit. Incidentally SwRI also designed the pumping system for the Sabatier reactor aboard ISS.
However, higher pressure and similar temperature GE-designed steam / coal-fired plants are already in operation in Germany:
The new miniaturized turbine technology could be useful for desalination plants, too:
I seem to recall that we also have a high salinity ice problem to resolve for our prospective Martians.
Offline
Like button can go here