New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2018-11-11 23:42:02
Re: Steam powered rovers
I want to follow up the previous post by talking about how different cycles and functions are different from the steam turbine cycle (Technically known as the Rankine Cycle) described above.
The first modification I want to talk about is the use of pistons instead of turbines. Pistons are simpler and work better at lower power, but they're also less efficient: While a turbine might achieve 85% of the isentropic (ideal) work, a piston is probably closer to 70%.
The second is a rocket engine, which is technically a thermodynamic engine. In this case, the working fluid heats itself via combustion. The work input comes from tapping the output directly, to power a turbopump, and the work output comes in the form of kinetic energy in the exhaust. There is no cooling stage; instead, the fuel cools as it mixes with the atmosphere.
The third is an internal combustion engine. In an ICE, the compression happens in part by mechanical compression by the piston, but a lot more compression happens because of the combustion, which produces extra gas and massively increases the temperature of the gas inside the fixed (or, quasi-fixed, relative to the speed of the piston) volume of the cylinder. Like a rocket engine there's no cooling phase because the gases are vented to the environment and allowed to cool there.
The fourth is a heat pump. Most often, a heat pump is used to remove heat from a volume, which is called refrigeration. For a heat pump, you start with a gas and heat it by compressing it (By applying work). You allow this heat to leave the gas, then allow the gas to expand while recovering some work. Then, the gas picks up heat from its environment, after which you restart the process.
The fifth is the system kbd512 and I have been discussing. In this system, compression happens via freezing CO2 out of the atmosphere and then reheating it to melt and pressurize it. The work input is the work that goes into operating the freezer. The heat input comes mostly from the environment (this is the heat that is used to vaporize the LCO2) with additional heat coming either from a Radioisotope Heating Unit or from freezing water. Work output comes from either a turbine or a piston engine (whichever is chosen) and there is no cooling stage.
As promised, I'm going to go more into detail on this system in this post because I have new information available in the form of thermodynamic data for CO2.
So, as I mentioned previously the heat of sublimation is 571 kJ/kg for CO2. This is the energy that will need to be removed from the atmosphere in order to freeze it out. With a COP of 4, this will cost 145 kJ/kg of work input.
Some of that heat will be returned to the LCO2 to melt and pressurize it. This heat is "free" because I already accounted for the energy cost of transferring it.
Next, there is the energy required to vaporize the LCO2. This is to be provided from the environment, which is at a higher temperature than the LCO2. I do have some concerns about how efficiently this energy can be obtained. Where does it come from? The ground is generally not a great conductor of heat, and the atmosphere is worse. It wouldn't be difficult to get to an adequately high temperature using sunlight (put a black surface behind some glass then fill the space with water, or perhaps methanol/ethanol if you're concerned it may freeze), but then you're dependent on sunlight, which means you can only operate during the day. It'll take some time to warm up, too. The energy to vaporize the LCO2 is about 330 kJ/kg, by the way.
Then there's the additional energy used to superheat the CO2. Heating to -20 C will cost you about 30 kJ/kg, while heating to 275 C will cost you about 425 kJ/kg.
From there, my textbook fails me. It does not list thermodynamic properties for temperatures below -50 C. However, by comparison to water vapor I determined that Cp∆T is an acceptable (though imperfect) proxy for the change in enthalpy.
I want to point out that this is a different calculation than the one I was doing earlier. Earlier, I was approximating the work done using the equation W=(Cv-Cp)∆T based on an incorrect interpretation of the first law of thermodynamics. I am not entirely certain why it was incorrect but the W=Cp∆T method accords better with the available data and is consistent with the procedure I laid out above.
Anyway, Cp is a little tricky because it varies with temperature. Luckily, the NIST WebBook provides the coefficients for an equation. I integrated this equation and put it on a spreadsheet. Feel free to use the spreadsheet: Here's the link. All the cells except for Tc and Th are protected, but the "W" cell is the energy output from the turbine. My recommendation for this spreadsheet: Enter your parameters in the yellow, look at the results in the green, and ignore everything else.
So: For a hot temperature of -20 C and a cold temperature of -75 C, the work done is 41.4 kJ/kg CO2. For a hot temperature of 275 C and a cold temperature of -75 C, the work done is 318.6 kJ/kg CO2.
If you wanted to do a reheat cycle with a max temperature of -20 C, you will need to do 4 cycles total, including the first one which I have already described. The total heating input will be 154 kJ/kg and the total work output will be 166 kJ/kg.
The total efficiency for a hot temperature of -20 C with a single stage is -28% (the system consumes more energy than it produces). For a hot temperature of -20 C with four stages, the efficiency is 4.3%. For a hot temperature of 275 C, the efficiency is 23%, which is not terrible.
Having said that, this may be a case where efficiency doesn't matter. This is fundamentally an energy storage system, after all. Every battery has a negative efficiency. If your hot temperature is -20 C, your heat source is so low-grade and simple that you might not care if your efficiency is terrible. Likewise, if your energy is coming from radioisotopes your rate of power usage is really much more important than the efficiency of the system.
For consistency's sake, I will also give an efficiency number comparable to the one I gave before. This one ignores the work input to the freezing units and the heat required to vaporize the LCO2 (the former being provided at a different location and the latter obtained for "free" from the surrounding environment). For -20 C with a single stage, this efficiency is 135% (the system produces more usable energy than is input in the form of heat). For -20 C with 4 stages this efficiency is 108% (the system still produces more usable energy than heat input, but it's much closer). For 275 C this efficiency is 75%.
Every efficiency number provided assumes ideal isentropic efficiency for the refrigerator and the turbine/piston, which naturally won't exist in the real world.
So here's the outstanding questions for this system:
Is it reasonable to try to vaporize CO2 using heat from the environment?
How does your required power consumption compare to the available power you can get from an RHU?
What's more important: On-site efficiency or total system efficiency?
Is it worthwhile to generate jars of LCO2 and carry around or store them for later use?
-Josh
Offline
Like button can go here
#27 2018-11-11 23:43:34
Re: Steam powered rovers
According to Wikipedia (https://en.wikipedia.org/wiki/Entropy), it's essentially the number of states in a given system that are consistent with the given parameters (pressure, temperature, etc.), which I think are finite but really hard to count. Apparently it's the natural log of that multiplied by the Boltzmann Constant, which has J/K units, hence entropy has J/K units (while entropy/mole is J/molK).
I must admit that I still don't quite understand how this ends up being so significant in so many fields but I guess I'm closer to understanding what it is.
-Josh
Offline
Like button can go here
#28 2018-11-12 10:07:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
These are simular equations as to how one removes power amplifier heat.
Offline
Like button can go here
#29 2018-11-12 14:27:37
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
I'm not sure how much use I'll be in helping resolve your outstanding questions, so I'll try to provide some info about the GPHS modules in our RTG's that could potentially provide the thermal power input to this system, in hopes that it will prove useful in some way.
First, let's examine cost. At some point, it has to be considered. It cost the American tax payers approximately $65M/yr to maintain the personnel and facilities infrastructure to eventually produce approximately 1.5kg of Pu238 per year by 2026 (current ORNL production rate is only around 300g to 500g per year), for NASA's more or less exclusive use. The total program head count for US DoE is around 135 full time government employees working at around a half dozen different facilities. Approximately $30M is directly related to Pu238 production and the rest is to pay salaries and maintain the multiple facilities involved in highly specialized infrastructure for materials and component fabrication. NASA must fund this DoE effort to the tune of about $50M per year. The case would inevitably be made that our expensive Pu238 is put to better use in science missions that require heat and/or power in places where available solar radiation is severely limited. They have a point. It's expensive to make.
There are alternative nuclear heat source materials such as Sr90 (165W/kg) vs Pu238 (571W/kg) that are essentially free nuclear waste products that reactor operators around the country might actually pay our government to dispose of for them. Sr90 is manufactured and separated by the truck load as a consequence of fissioning U235. The half life of Sr90 is only 29 years compared to 88 years for Pu238, but that's not necessarily a bad thing in this application. Sr90 is very well characterized, but not extensively tested for compatibility with existing technology infrastructure created and maintained at those aforementioned facilities specifically to provide power for space-rated systems, as the Pu238-based technologies already are. The primary benefit of using Sr90 would be total program cost, which would be limited purely to fabrication costs, since we can't run out of Sr90 without shutting down all of our commercial power reactors. By using Sr90, the total inventory of radioactive materials would be 3.46 times greater compared to Pu238, on a per-application basis, for equivalent power. For practical purposes, the heat source material choices are material property limited to alpha or beta emitters with decay products that aren't gamma emitters. The list of emitters with that property is pretty short. Only Sr90 is readily available in functionally limitless quantities and has a useful half-life. Am241 was studied by ESA, but like Pu238, Am241 is quite expensive to produce. Apart from the Voyager probes, all of our actual historic use cases or missions don't last beyond a decade or two.
Anyway, moving on to current Pu238 technology...
Each GPHS brick contains a total of 600g of Pu238 dioxide in 4 pellets that produce a combined 250Wt. Each 150g pellet is approximately 27mm by 27mm and produces 62.5Wt at time of production. The Pu238 pellet is sealed in an Iridium cladding with a pressure release valve. The Iridium is surrounded by a Graphite Impact Shell (GIS). Two of the cladded pellets are inserted into each GIS with a floating thermal expansion membrane between them. The GIS itself is sleeved with Carbon-Bonded Carbon Fiber (CBCF). Two of the CBCF assemblies are inserted into each General Purpose Heat Source (GPHS) graphite brick that's intended to withstand the heat of reentry and subsequent surface impact.
The surface temperature of the Pu238 ceramic metal is 1755K. The surface temperature of the cladding (I think this actually refers to the CBCF assembly, but I'm not certain) is 1144K. The surface temperature of the brick is 811K. The brick transmits the heat to the thermocouples and radiators that produce the temperature drop that creates electrical power using the Seebeck effect.
There's no functional reason why the heat sources for the Gas Tube Heater Assembly (GTHA, yes I just made up a new acronym) couldn't be simplified to only use the Iridium clad heat sources during actual use. Even hot Iridium is highly resistant to oxidation from CO2. The CBCF assemblies could be removed from the graphite aeroshells / bricks after the lander successfully delivers them to the surface of Mars, whereupon their Iridium clad heat sources could be extracted from the CBCF and GIS assemblies. That would protect the heat sources during launch and reentry using previous test and launch accident validated methods, but provide higher grade heat for the GTHA.
The GTHA will be an insulated Hastelloy tubing with channels or "fins" machined or sleeved into the tube. That material should maintain sufficient strength and oxidation resistance to reliably operate for years at high pressure and temperature and contend with temperature gradient (cooling) in operation. Someone smarter than me needs to figure out if it's more beneficial to spread lower temperature heat over a greater surface area by keeping the graphite shell (the GIS tube, not the GPHS brick) that would necessarily increase the GTHA diameter and weight or if it's better to remove the graphite shell and just retain the Iridium-clad heat source for use in the device. My USWAG, having calculated nothing, is that it's better to get higher temperature / grade heat to play with since the Hastelloy can take the heat. The completed device would weigh a few kilos and attach directly or via hose to the LCO2 cylinder on one end and the output goes to a hose (air tool) or piston engine feed pipe / fitting (vehicle) on the other.
Real World Considerations:
The GTHA is hand-safe, meaning an astronaut can pick up and handle the device without special thermal protective equipment, but the heat sources are capable of causing instant third degree burns. Along with the alpha particles, the Pu238 releases a small but measurable amount of radioactive Radon from impurities. Therefore, GPHS brick storage and disassembly is something that must take place outside of pressurized habitable spaces in a relatively clean environment. A field tent with a CO2 blow-down system would take care of the atmospheric dust, for both the astronauts and routine maintenance of their GTHA devices. There are also some light Gamma emissions from the other isotopes mixed in and neutron radiation from Pu238's spontaneous fissioning. The Iridium attenuates most of the gamma, but has little effect on the neutrons. 81% pure Pu238 (I forget what the actual purity of the stuff that NASA uses happens to be, but I think it's in the mid-80's) produces 2.2K neutrons per gram-second of material. The critical mass of a bare sphere of Pu238 is 10kg and the critical diameter is 97mm, so putting all of your heat sources in a pile is not advisable even though the stuff is nowhere near isotopically pure. Any Am241 impurities in the Pu238 source will lead to the creation of Pu236 daughter products, which are strong gamma emitters.
The Hastelloy in the GTHA would stop nearly all of the Gamma, to the point where the ambient environment is far more of a radiation threat than the GTHA. Those same problems also apply to Sr90. The heat sources shall therefore be handled outside in dust-limited CarbonX fabric tents / structures whilst using CarbonX gloves / gauntlets / sleeves that easily withstand the heat involved, enabling astronaut to insert or remove the GTHA heat sources without fear of thermal burns or Radon contamination of pressurized environments. Just to be clear, the gamma emissions are already substantually attenuated by the paper thin Iridium cladding and the Hastelloy in the GTHA provides additional gamma shielding that reduces those emissions to levels below background radiation levels.
There are no peaches and cream here, but Pu238 is still a heck of a good heat source. A Thorium fuel cycle in a commercial reactor would produce isotopically pure Pu238 in multi-kg quantities per 1,000t batch of U233 fuel and the cost would take a nose dive off a cliff, which would be really helpful. If only we could operate a single GW-class LFTR for a year... 15kg of Pu238 per fuel batch. NASA would have enough material for anything the tax payer could actually afford to fund.
After receiving training, do you think we could trust our astronauts not to arrange their heat sources in a configuration that could produce a criticality accident?
Should we just ship assembled GTHA's in graphite shipping containers instead?
At some point, they still require maintenance.
It's honestly not that dangerous if you don't do dumb things with it, but I'm sure Louis will have a field day with what I just admitted to.
Offline
Like button can go here
#30 2018-11-14 00:03:48
Re: Steam powered rovers
Hey kbd512,
My high-level take on radioisotope power is that it's good under the following circumstances:
Relatively low power (below ~1 kW)
Not used for primary generation
Reliability is critical
Cost is not a major concern
Alternatives are inferior to the point that it's worth getting into a political fight over nuclear power in space
A corollary of points 2 and 4 is that it's being used for a niche application.
It seems to me that 1 through 4 are the case in this application. Whether or not 5 is true is up to the organization running the mission, and in my opinion this is relevant whether that organization is public, private, or somewhere between the two.
To speak briefly on the politics of nuclear power more broadly: It is my opinion that nuclear power is a great source of electrical energy, particularly if you want to reduce CO2 emissions associated with electricity production. However, there is always going to be some tiny chance (no matter how good your engineers and procedures are) that some series of mistakes, equipment failures, laxness, and bad luck that will result in a release of radioactivity to the environment. The average person does not spend much time thinking about energy policy and the average person is certainly not an engineer. Radiation is scary but also a genuine threat to your health in large quantities that can neither be avoided nor repaired. I guess what I'm saying is that, to a certain extent, I get why nuclear is unpopular even if I personally think it should be deployed more widely.
On the topic of Pu-238 vs. Sr-90: I say go for whichever is more available, basically. I would like to see investment in the facilities to produce this because our need isn't going to go away. In the context of a serious exploration/settlement program $50 million isn't that much money and it's a great investment if it improves mission capacity or reliability.
As far as the question of whether to remove the outer layer of covering from the Pu-238 bricks: I wouldn't. There's some benefits if you're using a gas turbine or something, but really 811 K is really quite hot, especially against a background of 230 K. For carnot efficiency purposes, 811 K against a background of 230 K will be comparably efficient as 1023 K against a background of 290 K. There's no way the CO2 will get to a temperature even close to that and I don't think heat transfer will be a huge issue. Even if it does reduce effective power somewhat, bringing 6 RHUs isn't meaningfully more difficult than bringing 4.
On the other hand, a criticality incident (no matter how unlikely) is an avoidable mission-killer. The best case is that you've introduced another task that's potentially hazardous and requires specific attention to details. This is true no matter how simple it would be to avoid a criticality incident--it's best for it to be a fun piece of trivia rather than a real procedural consideration for the astronauts. The worst case is killing or stranding one or more crew members.
I've already said it plenty but I want to reiterate for the crowd that I agree that a criticality incident is both extremely unlikely and extremely avoidable.
-Josh
Offline
Like button can go here
#31 2018-11-14 05:28:03
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
Thanks for the input. After thinking again about the worst thing that could possibly happen, you're correct in your assessment that the heat sources should stay in their impact shells. I didn't want stray atmospheric debris in the LCO2 to become lodged in the porous shells, but a slight redesign could fix that. And yes, even the impact shells are really hot. I'd like to say that we could trust the smartest people we can find to not do inadvisable things with their heat sources, but common sense is in short supply these days and I don't want any accidents to injure or kill people who may not fully understand what they're doing on account of not being nuclear engineers.
Offline
Like button can go here
#32 2018-11-14 10:18:00
Re: Steam powered rovers
I wouldn't even call it common sense, exactly. Nobody can pay attention to all things at all times, people get tired, and don't always make the right decisions in fast-moving situations, etc. Going to Mars is already going to be an extremely difficult task for the crew, so we might as well remove a bit of tail risk where we can.
"Design is for the user, not the designer" is something someone said to me once that really stuck with me.
-Josh
Offline
Like button can go here
#33 2018-11-14 21:18:26
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
Well, it seemed like common sense to me that if you have nuclear materials on hand that could create a criticality accident if configured in a way that permitted that to happen, then you shouldn't arrange them into a critical configuration. In simple terms, you handle one piece of material at a time and you keep them physically separated from each other. That should be pretty easy to remember. When I take a tray out of the oven, I don't need to remind myself that the tray is hot. Then again, I've also burned myself on hot aircraft engines thinking "I can reach that thing that I dropped without getting tattooed", but I didn't, so I understand where this is going. In light of that, I agree with the wisdom of not putting people into situations where they could get hurt to avoid the danger altogether. The factory is also much better equipped to handle radioactive materials than an astronaut ever could be.
Offline
Like button can go here
#34 2018-11-14 23:32:33
Re: Steam powered rovers
Well that's the thing, eh? 99.8% of the time you don't get burned. All you've got to do is not touch the hot pan with your hand. Common sense, right?
Anyway we agree so there's no need to beat this horse any further.
How do you feel about using liquid CO2 and liquid water for heating for nighttime energy storage? It seems to me like a really good option (highly efficient, reliable, easily manufactured in situ) but I'm interested in your take.
-Josh
Offline
Like button can go here
#35 2018-11-15 19:34:06
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
I could definitely see an application for base power at night to preclude the need to take so many batteries or burn precious rocket fuel.
What did you have in mind (specific application, power output level, general system configuration, estimated system mass, etc)?
Is this something that could be implemented without microelectronics, even if the overall efficiency isn't the absolute pinnacle of what could possibly be achieved?
Offline
Like button can go here
#36 2018-11-15 20:45:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
These sound sort of along the lines that Josh is suggesting
Offline
Like button can go here
#37 2018-11-15 21:05:25
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
SpaceNut,
I meant something along the lines of this:
Application: base power, base combined heat and power, propellant plant power, etc
Power Output Level: several kWt and kWe or something substantially more than that
General System Configuration: primary system components, configuration of the heat transfer loops, solar concentrator water heater, etc
Estimated System Mass: <2t (a limitation on the crane aboard BFS, not necessarily what could be landed)
Offline
Like button can go here
#38 2018-11-15 22:04:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
The hot water that was created from the heat pump system then would be used at night to make the same phase change compression of co2 from dry ice to create power much in the same manner that we are looking to do with a solar chamber or other sources of heat.
Offline
Like button can go here
#39 2018-11-15 23:01:48
Re: Steam powered rovers
I'm thinking primarily about early to not-so-early settlement phase, more than the first mission, but I guess in principle it could be used for either.
Power production would be in the range of ~500 W or more (by comparison to the output of a very small internal combustion engine). There's no real upper limit and the system will get more efficient the bigger it gets. I estimated above that a LCO2 tank will mass 25% of what's inside it, but for a settlement you might be able to get some savings by burying it deep underground. For reference, a 10 atm pressure corresponds to burying a tank roughly 70 m below the surface of Mars. Seems like a lot to me but I suppose it's not impossible if you're working at a very large scale. I suppose you'll get temperature stability if you're buried that deep.
I'm not sure how much the refrigeration, power production, and water heating/storage would weigh. I think this is the kind of system that makes more sense for a time when you're building things in situ than for an exploration mission. But I also don't exactly understand the problems you believe there to be with batteries (presuming the initial mission is solar powered, which is far from settled but definitely possible).
Here's how the system would work:
Freeze Carbon Dioxide out of the atmosphere: This involves removing about 600 kJ/kg of energy from the CO2. Depending how cold your hot side is, this requires an input of about 150 kJ/kg of work plus inefficiency losses. If you get clever with your hot side, you can maybe do a little better. If you're feeling really clever maybe you can find something to freeze at night and then use as your hot side during the day to improve your efficiency.
Sponge up some heat. What I have in mind is using solar energy to heat water up to room-temperature or so. You might even use it as the "cold side" of your main generator (which, by the way, if we're talking about solar power made on Mars I'm a big fan of the solar power tower). If you don't get to cheat by getting your waste heat from somewhere it's trivially easy to build a solar power collection system that can heat water to 20 C on Mars.
Use some waste heat from somewhere (such as the heat rejected at the hot side of your refrigerator unit) to melt the dry ice into Liquid CO2 and pressurize it. The reason why I would say to freeze it and then melt rather than going straight from gas to liquid is that it saves you the difficulty of pressurizing gas from 0.007 atm to 5 atm (an increase of over 700 times) which would probably cost much more energy.
Store this liquid CO2 at an appropriate pressure and temperature (somewhere around 5-10 atm, -55 C to -40 C probably) until nighttime
Use lukewarm water to boil the CO2 and expand it through 4-5 reheat cycles (As described above) at night to extract useful work, eventually ending up with a block of ice and releasing the CO2 to the atmosphere to generate work.
In the ideal case, using the calculations in my post above (post #26 in this topic), the system would consume 145 kJ/kg of work in refrigeration, 650 kJ/kg of heat energy (Which corresponds to 2 kg of lukewarm water to each 1 kg of CO2), and would output 166 kJ/kg of useful work. The actual refrigeration work will be higher and work output lower, unfortunately.
In principle, the technology that could be used is what Zubrin likes to call "gaslight era". Seeing as we do not live in that era there's all sorts of electrical and electronic sensors, valves, etc. that can improve the efficiency and reduce the labor required for operations, but it's not required for the system to work.
A funny, steampunk-style image that crosses my mind is workers in spacesuits shoveling dry ice like coal into a tank.
-Josh
Offline
Like button can go here
#40 2018-11-17 14:40:00
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
I like the concept since it uses local resources that we should never run out of, but I need some mass numbers for the heat exchanger and the pump. Tank masses are easy enough to calculate. Although all of the SCO2 heat exchanger subsystems in power plants are one-off designs (mostly geometry differences and sometimes material differences), we still need ballpark figures (1t or 5t for a 500W system?) to explore the idea further. Use industry standard materials. The SCO2 systems appear to favor the use of a combination of stainless steels and Titanium alloys to contain CO2 under significant pressure. The advantages are that the systems last for decades with minimal maintenance. The downside is the mass and cost of the materials. Other materials could be substituted, but the body of knowledge from industry will use specific materials and design practices that are known to work well.
I'm not a fan of the idea of using design tricks such as burying CO2 tanks underground to save on system mass. I'd much rather that the system was installed at ground level in a pit or crater near the solar power tower to contain any potential failures. Compared to molten salts, which can remain hot enough to cook with more than 24 hours after initial heating, water is a comparatively poor thermal storage medium. It's primary advantage is that it's available on Mars in mass quantities.
Offline
Like button can go here
#41 2018-11-17 17:52:25
Re: Steam powered rovers
I agree that it doesn't make too much sense to bury the tanks, at least not initially.
Having already calculated the tank mass for CO2 I think we already know enough to say that the system will be too heavy to be worth importing. I calculated in post #17 in this topic that a spherical tank designed in accordance with standard engineering practice and made from 7075 Al would mass 250 kg/m^3. LCO2 has a density of 1100 kg/m^3, so the tank will be 23% of the mass of what's in the tank. This is an absolute minimum: It doesn't include the valves/tubing, the refrigeration equipment, the equipment used to melt or store the water, or the piston/turbine assembly.
In a quadruple reheat cycle, you get 166 kJ/kg of useful work (minus inefficiency losses) per kg of CO2. This corresponds to 0.046 kWh/kg of CO2, and 0.2 kWh/kg of tank. The other components will mass at least as much as the tank.
So, for example, to sustain 1 kW for 10 hours will require 200 kg of tank alone. To sustain 50 kW overnight would be at least 3750 kg of tank, plus probably a substantial amount of other equipment.
-Josh
Offline
Like button can go here
#42 2018-11-17 21:57:24
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
Josh,
How long could a 10t to 15t CO2/H2O heat pump produce 50kW without significant maintenance?
If Lithium-ion batteries only last 2 to 4 years before requiring replacement due to environmental and duty cycle degradation, but this thing would last for 20 years or more and require no electronics, then perhaps simplicity and longevity and the confidence that no amount of electronics glitches / software crashes / solar flares / computer hacking by infantile nitwits can ever take base power offline is more important than initial delivery tonnage.
NASA says 40kWe continuous is the power requirement for a small base of operations. If this thing runs into perpetuity without constant fuss, then this is the way to go. Everyone loses their minds of nuclear power, but this thing is only about double the mass of the 4 to 6 40kWt/10kWe KiloPower units that NASA is building, nobody will freak out about launching it, and any Joe can operate it without a license and approval from US DoE.
Offline
Like button can go here
#43 2018-11-18 10:51:44
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
Post 6 of this topic was also posted about in the running on compressed air topic http://newmars.com/forums/viewtopic.php … 04#p142504
https://www.irjet.net/archives/V5/i9/IRJET-V5I913.pdf
WASTE HEAT RECOVERY BY THERMOELECTRIC GENERATOR AND SOLAR PANELS FOR COOLING AND HEATING EFFECT BY PELTIER
https://www.seas.upenn.edu/~lior/docume … lished.pdf
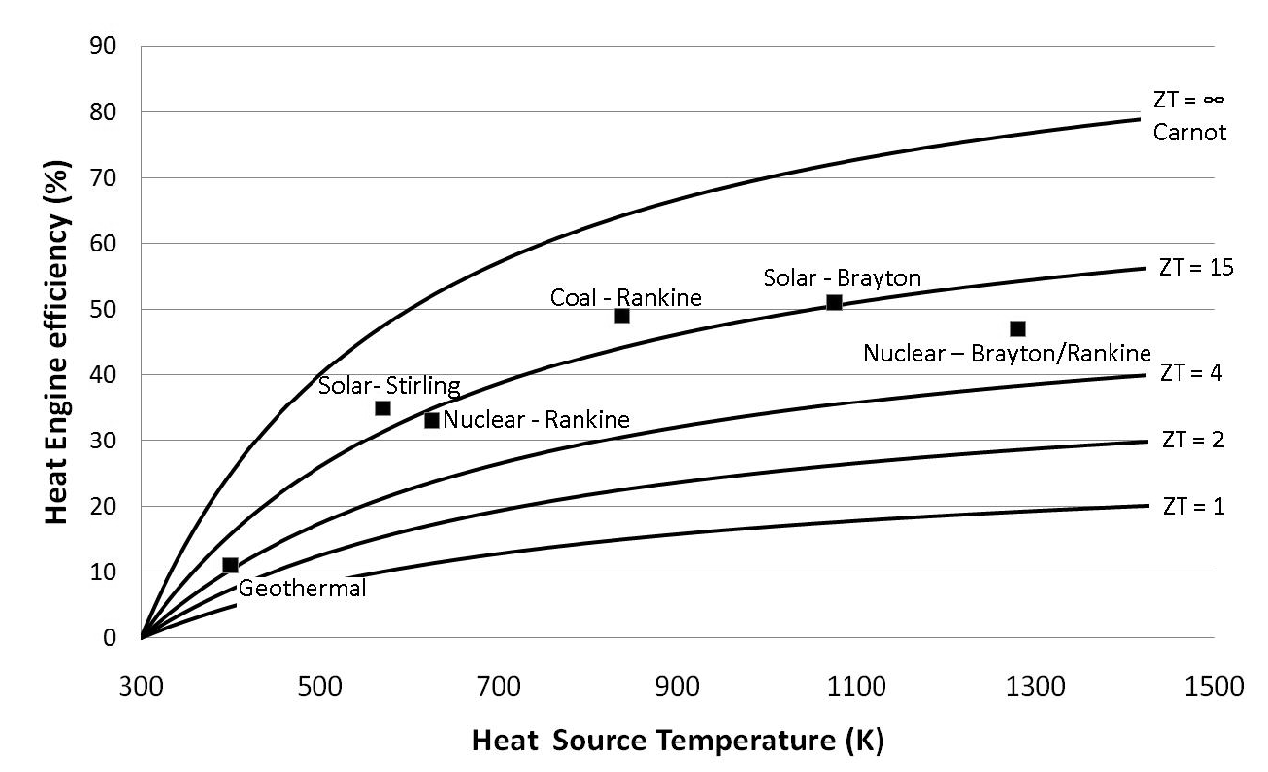
Analysis and comparison of solar-heat driven Stirling, Brayton and Rankine cycles for space power generation
http://www.unimasr.net/ums/upload/files … 0d4219.pdf
APPLIED THERMODYNAMICS (PART I) SECOND – LAW ANALYSIS OF ACTUAL PROCESSES
https://www.seas.upenn.edu/~lior/docume … npress.pdf
Offline
Like button can go here
#44 2018-11-18 11:54:06
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
No matter where the watts come from—grid, fuel generator, solar panels, wind, other renewables (a watt, is a watt, is a watt!)—the ironclad rule of power generation applies: power generated in the circuit must be greater than or equal to the power that will be consumed in the circuit.

https://animatedscience.co.uk/?s=KE+ani … arch_404=1
buried in another power topic
http://theconversation.com/how-energy-f … mars-38250
http://www.elkraft.ntnu.no/eno/Papers20 … thesis.pdf
Offline
Like button can go here
#45 2018-11-18 15:56:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
Offline
Like button can go here
#46 2018-11-18 18:42:37
Re: Steam powered rovers
On reliability:
I haven't figured out exactly how I think the freezer system should work. There's a couple big challenges with that part. The first challenge is the low density of the Martian atmosphere: To get a single kilogram of CO2 you'll need to process roughly 65 cubic meters of Martian air.
The second is the heat transfer, specifically how you get dry ice in a useful form (i.e. not as frost on your cooling element).
Presuming this can be handled in a simple, reliable way, the next least reliable system will be the freezer. Freezers are in general pretty reliable and in general have long lifetimes (how old is your oldest refrigerator?).
One thing worth pointing out: The design pressure for the tank I specified was 10 atmospheres. You could halve the mass by reducing the design pressure to 5 atmospheres. I also specified a safety factor of 5, which is standard for Terran pressure vessels but could be reduced in principle.
-Josh
Offline
Like button can go here
#47 2018-11-18 18:53:50
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,518
Re: Steam powered rovers
SpaceNut,
In the third article referenced in Post #43, that was more along the lines of what I had in mind for solar power on Mars, rather than using an open cycle. The 50kWe system for Space Station Freedom was supposed to have a total mass in the 5,500kg to 7,500kg range, dependent upon what cycle and gas was used. I'd prefer CO2 since there's plenty of that on Mars, even if the total mass increases. Each 25kWe SDPE (this was actually built in 1987 by SunPower for NASA; note that SunPower is still a contractor that makes Stirling engines for NASA, but IIRC the bid for the Stirling engines in KiloPower went to Qinetiq) free piston Stirling engine had a mass of approximately 137.5kg. Just in case KiloPower doesn't pan out or some politician kills the program, I'd like to have a solar backup ready to take over.
That Mini BFS concept could aerobrake to land on Mars, open the payload clamshell, deploy the concentrators and radiators, and test our ability to start making LOX at rates that actually matter. If the system is successful, then a hybrid of that system could provide base power and power for LCH4 production when coupled with hot H2O and LCO2 storage from Josh's concept.
Offline
Like button can go here
#48 2018-11-18 19:36:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
Open chamber to allow frost to form on slide out plates and if not cold enough close side panels and turn on the refrigeration. That get moved before sun rise to the pressure creation chamber to which the co2 would melt and then would allow for collection into a tank for later processing. Once collection is done repeat until we have all the co2 we want. All of this could be solar panels and batteries for making it function automatically.
Offline
Like button can go here
#49 2018-11-18 22:28:39
Re: Steam powered rovers
The biggest problem with freezing CO2 is volume. A system able to generate 50 kW at night probably needs to be freezing 600-1000 g per second of CO2 during the day, which is a substantial volume of atmosphere. I'm not sure exactly what the right way to do it is.
-Josh
Offline
Like button can go here
#50 2018-11-18 23:38:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,626
Re: Steam powered rovers
https://en.wikipedia.org/wiki/Martian_polar_ice_caps
Part of the ice cap consists of dry ice, solid carbon dioxide. Each winter the ice cap grows by adding 1.5 to 2 meters of dry ice from precipitation from a polar-hood of clouds. In summer, the dry ice sublimates (goes directly from a solid to a gas) into the atmosphere. During each year on Mars as much as a third of Mars' thin carbon dioxide (CO2) atmosphere "freezes out" during the winter in the northern and southern hemispheres. Scientists have even measured tiny changes in the gravity field of Mars due to the movement of carbon dioxide.
http://www-mars.lmd.jussieu.fr/mars/pub … _tobie.pdf
Numerical simulation of the winter polar wave clouds observed by Mars Global Surveyor Mars Orbiter Laser Altimeter
https://en.wikipedia.org/wiki/Carbon_dioxide
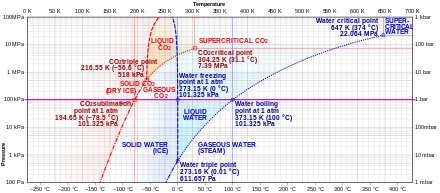
Once co2 is collected the system can be built to have a large low pressure gas capture recycling tank to which we can recollect it for reprocessing.
Offline
Like button can go here