New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2018-11-08 13:41:05
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,236
Dry ice pneumatic tool
This topic might be of interest to void, because of many "dry ice" search results.
I searched for "dry ice" and for "mechanical engine" on the forum, and got too many hits.
The article may have already been entered here, but if so i did not find it.
The article at the link describes a high school science project work supervised by a retired NASA engineer.
It reports operation of a pneumatic tool using sublimed dry ice as the working fluid.
http://marsforthemany.com/news/technolo … ce-engine/
Chase Bishop and James Thompson.
Based upon the date of comments, this work appears to have been done in 2017.
(th)
Last edited by tahanson43206 (2018-11-08 13:42:04)
Offline
Like button can go here
#2 2018-11-08 19:31:53
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
Another possibility is harnessing temperature change to use frozen CO2 as a power source...
Here's a link explaining how it works:
We have looked at the issues of pressurization and it was noted that natural power of the sun to warm the dry ice would give aid to man in its collecting of it for making fuel.
Offline
Like button can go here
#3 2018-11-08 19:33:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
Its funny how we will create multiple topics and they will drift and mix...
Using CO2 expansion would be a lot more viable source of motive power than steam, as a function of achievable power-to-weight ratios. Small vehicles such as motorcycles could easily use CO2 cylinders (Scuba tanks) to power piston engines, for example.
Like so:
Offline
Like button can go here
#4 2018-11-08 20:02:48
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,334
Re: Dry ice pneumatic tool
SpaceNut,
I think CO2 powered tools and light vehicles are the right technologies for construction tools (not construction vehicles) and base transit on Mars. In industry, pneumatic or hydraulic tools are used when substantial power is required in a lightweight package. Fine machining operations are typically done using computer-controlled electric motors (CNC mills, lathes, 3D printers, etc), but construction tools only use batteries for convenience. There are some exceptions, but an air drill is typically lighter than an electric drill of equivalent horsepower and lasts substantially longer in high duty cycle environments, therefore easier to use for hours on end. Which is "best" mostly comes down to the expected duty cycle of the tool. A high duty cycle tool should probably be CO2 powered. A comparatively low duty cycle tool could be electric or manual (no batteries or electronics to fry). CO2 powered tools typically require more user-maintenance than electric tools, such as daily disassembly, inspection, cleaning, and lubrication to inhibit corrosion (should be far less of a problem on Mars with so little water vapor in the atmosphere) and excessive wear. It only takes a few minutes, but must be done.
Offline
Like button can go here
#5 2018-11-08 20:51:29
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
The tools for mars if using earth pressures means an issue if we are having issue getting there from the thin mars air.
How To Maximize Air Tool Performance Part #1 | Pressure and Flow
I am reminded on running on compressed air topic....
Offline
Like button can go here
#6 2018-11-09 08:18:48
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,236
Re: Dry ice pneumatic tool
While looking for information about location of dry ice on Mars, I found this report from NASA:
https://www.nasa.gov/content/dry-ice-moves-on-mars
In American history (and likely other nations) there was a time when humans could earn income sufficient to sustain entire communities by collecting ice during winter months and storing it for cooling purposes during hot weather. Ice was (apparently) even shipped overseas for a time. Something similar seems possible for economic activity on Mars. I would guess that families or small companies might earn a decent return by collecting dry ice and returning it to regions where crops are grown for mechanical power application.
I tried searching for the word "economy" and got 114 pages of results. Someone may already have discussed the economic potential of dry ice collection, but the search term "economy" does not appear to be a good way to find it.
(th)
Offline
Like button can go here
#7 2018-11-09 10:22:12
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,031
Re: Dry ice pneumatic tool
Nice Topic.
I would start here, the solubility of gasses in water.
https://www.engineeringtoolbox.com/gase … _1148.html
I am presuming that their graphs are based on Earth sea level pressures, and fresh water.
This material would fit into atmospheric separations also, but it is a current discussion. Perhaps I will place some of this material there as well.
In particular, the components of the Martian atmosphere as it actually is would be important.
https://en.wikipedia.org/wiki/Atmosphere_of_Mars
Quote:
Martian atmosphere composition
The Martian atmosphere consists of approximately 96% carbon dioxide, 1.9% argon, 1.9% nitrogen, and traces of free oxygen, carbon monoxide, water and methane, among other gases, for a mean molar mass of 43.34 g/mol.
Where the graphs on solubility of gasses in water goes down to the freezing point, they do not depict the solubility of these gasses in brines. That could be a double edged sword. Colder water should lead to greater solubility, but the salts of brines may crowd gasses out.
So, that needs to be explored. Different salts may have different effects.
This recent article supports the dissolution of Oxygen in very cold brines.
https://arstechnica.com/science/2018/10 … obic-life/
…..
To get more direct, my idea would be to compress Martian atmosphere in a chamber where it can be mixed with brine with optimal temperature for our purposes, and to speculate on the results.
My belief is that much CO2 will dissolve. Not all of the Argon, and Nitrogen I think. And most likely all of the Oxygen and Carbon Monoxide will dissolve, because there is so little of Oxygen and Carbon Monoxide. It is also important to keep in mind, that each gas will be interfering with the ability of the other gasses to dissolve. There is only so much room for gasses, so they will exhibit altered behaviors when other gasses are competing. However CO2 seems to be the most prone to dissolve.
…..
It is likely that we will get a sub-product here, possibly only mostly including undissolved Argon and Nitrogen.
We may have an opportunity here to run a CO2 steam turbine by then heating the solution, and expelling CO2 to gas by that process. The presumed source of heat being solar, but maybe nuclear.
The exhaust would most likely be >>> 96% CO2.
However for the purposes you have worked with I would rather degas the solution with a partial vacuum, and then pressurize it and cool it to liquid. It is not clear if the tiny amount of O2 and Carbon Monoxide end up dissolved in the CO2, or stay a gas. If a gas, then that could be used for chemosynthesis possibly.
Liquid CO2:
https://en.wikipedia.org/wiki/Carbon_dioxide
Quote:
Melting Point: −56.6 °C; −69.8 °F; 216.6 K (Triple point at 5.1 atm)
So you would need a pressure vessel for at least 5.1 atm. Probably you would make it stronger that that.
The Melting temperature seems rather close the typical average Martian environment temperature. I would think storage would be kept in shade, and with some thermal insulation.
So, then perhaps solar energy to warm it up to drive engines. That's enough for now.
……
If you want to avoid high pressure storage of liquid CO2, then you could flash off some of the CO2 to a gas and leave behind dry ice. Presumably you would prefer to recycle the gas. Also you could cool the liquid CO2, to produce more dry ice and less gas before flashing it, if that is what you want. Then of course you would sublimate the CO2 to a gas, probably using the heat of the day and solar energy, or some other heat source.
……
I have speculated on a gardening robot for Earth, where the robot would kill weeds by squirting freezing CO2 on the weeds. Less grunt work for humans, less toxins. But you would not want this thing to hurt humans. In this case for Earth liquid Nitrogen might be better as it is colder, and will stay liquid at our atmospheric pressures long enough to do huge damage to the weeds.
…..
George Church wants to revive a hybrid of Mammoths and Asian elephants. But I also think that such a Nitrogen squirting robot could also assist in killing small trees and bushes at high Earth latitudes. The reason to do this is that such trees are likely contributing to global warming. We may want to revive the Mammoth Steppe, as it is much more productive than the Taiga & Low Arctic Tundra "Savana/Marshes" that are believed to have displaced productive grasslands after the Mammoths went extinct.
Done.
Last edited by Void (2018-11-09 11:10:23)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#8 2018-11-09 18:39:30
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
The heat source could be pebble reactor, rtg or even a kilowatt reactor as we are not just looking for the heat source but also some electrical to make things all work together. Josh believes that this can be a low level heat source to make the dry ice go through phase change to gasseous and be pressured enough for use. I just do not know....
I do not know how well the water would work to capture co2 ..
Offline
Like button can go here
#9 2018-11-09 19:39:00
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,031
Re: Dry ice pneumatic tool
Actually it might do rather well, but I have not a problem with better.
But we can presume water and salts and cold temperatures on Mars, and CO2 very easily dissolves in cold water.
But do better by all means. I have no problem with better. Always want better.
But having water, salts, and cold on Mars + an atmosphere dominated by CO2, and knowing that CO2 dissolves rather well in cold water, it is a beginning.
One criticism I might politely offer to many of the members on this site, is that perhaps you are so concentrated on precision, that you have trouble seeing the forest for the trees, so to speak.
Rocket science gives little permissions. To get up from the Earth to space is a very narrow window. But for other things you need to approximate first, find the general area of possibilities and then close down on a precise best option.
It is as if you have a narrow vision which works very well for what it works for, but then you don't see the entire sky. You don't look for the other possibilities, because you cannot view them.
There are other CO2 capture methods that will be and are being developed for greenhouse gas reasons on Earth, and if they are better then so lets do better. But can you for an economy do it on Mars?
That will be the true decision. What works best on Mars.
Starting with dissolving CO2 in water/brine, we have a benchmark to make a evaluation of relative cost/productivity.
The deal with brine is that you can make it cold at night, and then process CO2 with it by solar energy during the day. Many other systems will not so easily adapt to those facts.
And remember we have not explored the pressure values. While the graphs are presumed for 1 bar, other higher pressures could be used and would induce a greater dissolution of CO2.
Done.
Last edited by Void (2018-11-09 19:50:15)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#10 2018-11-09 20:38:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
Pumping the mars air into a chamber filled most likely partially with brime but keeping the air warm enough to not freeze the brime as it becomes loaded with co2 forming carbonic acid.
This is what a smoke stack scrubber does and on earthwe are dealing with sulfur dioxide, nitroxides and other stuff that we do not want in our air.
How Do Smokestack Scrubbers Work?
amine solutions are used here on earth.
events.awma.org/files_original/ControlDevicesFactSheet07.pdf
www.mne.psu.edu/cimbala/me433/Lectures/MIT_smokestack_scrubber_for_CO2.pdf
Offline
Like button can go here
#11 2018-11-09 23:03:15
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,031
Re: Dry ice pneumatic tool
Yes, but that is on Earth. I really hate the binary reptilian brain thinking that happens on this site.
You insist on doing everything in accordance with what happens on Earth, what works best on Earth, and you always do a binary comparison and then eject any innovation. Your thinking is circular, and you will never, never be able to adapt to Mars.
You say greenhouse or dome as if it is a technological solution. It is only a word.
Things like that.
You spew propaganda, and ignore evidence.
It is tiresome. Think with your higher brain, and do better than binary. Perhaps scientific thinking is often done with a hostile method, but I think that is stupid. It shuts off the higher brain.
Quit penis fencing, try thinking.
I really am done.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#12 2018-11-10 11:18:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
A fish tank bubbler with a twist.
Starting with dissolving CO2 in water/brine, we have a benchmark to make a evaluation of relative cost/productivity.
The deal with brine is that you can make it cold at night, and then process CO2 with it by solar energy during the day.
Cost does not matter on mars only for launch of materials from earth and only productivity of the device will matter on mars.
Mars has no surface brine which is still up for debate as its not directly observed as staining only from slopes as seens from orbit.
That means a chamber of sorts will be made to make the device work, which you have indicated as glass clear in nature for use of solar warmer to make co2 exit the brine.
The average temperature on Mars is -80 degrees Fahrenheit. The temperature on Mars can fluctuate greatly due to the planet's inability to retain heat energy. Temperatures can reach up to 70 F at the equator during a summer day and as low as -195 F at the poles during a winter night.
http://marsnews.com/the-planet-mars
Any brine will eventually freeze if concentration of salts to the cold temperature of the entering CO2 which means only daytime operation of the device or warming of the entering co2 is possible. Which means a single layer glass chamber will not do as its got to be temperature isolated from mars.
We know that salty water, or brines, could remain in a liquid state at or just below the surface of the planet, as a water mixed with salts has a lower freezing temperature than plain water. Which is the increase pressure load of the soil and isolation form the surface temperatures that would make it possible.
Water on mars in a chamber still needs presurization to stay in liquid form which means the inlet pressure of the entering co2 must be greater than that value.

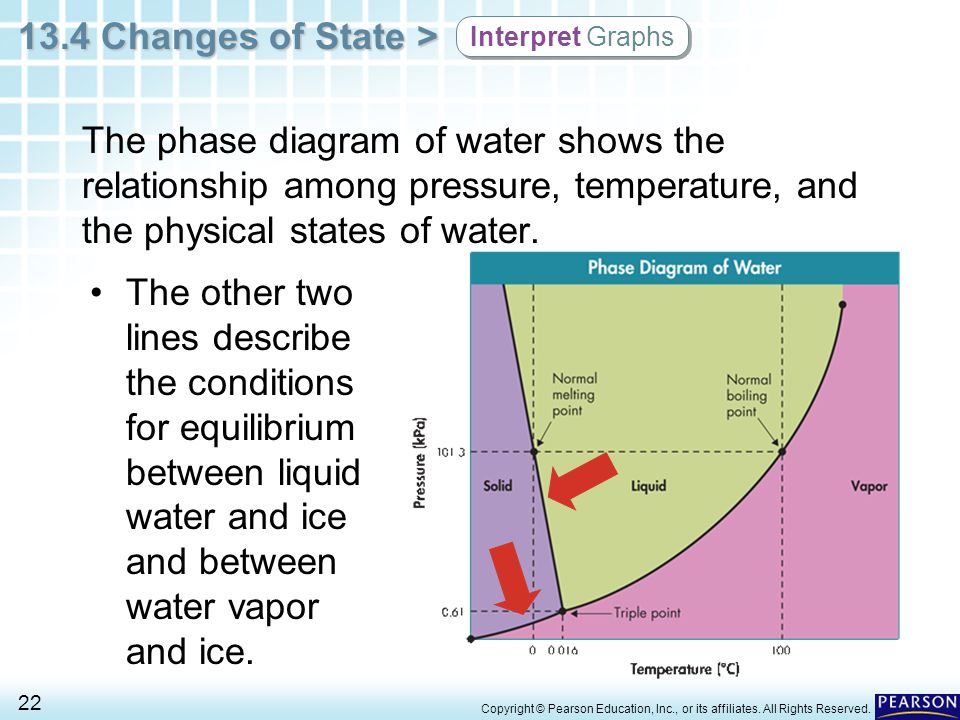
Mars could have enough molecular oxygen to support life, and scientists figured out where to find it
A new study suggests that salty water at or near the surface of the red planet could contain enough dissolved O2 to support oxygen-breathing microbes, and even more complex organisms such as sponges.
Mars’ atmosphere is extremely thin — 160 times thinner than Earth’s atmosphere.
Here is the water temperature with salts on earth:
The effects on water with salt concentration:
Physical Properties of Seawater
Offline
Like button can go here
#13 2018-11-10 12:05:09
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
Aeration is a unit process in which air and water are brought into intimate contact.
Something like this would set in the salty water at the bottom of the chamber where the mars air would come into the chamber:
See how this type of float control value works
Now you can also use porous stone but will need a oneway check value to keep the water from going backing into the feed.

Offline
Like button can go here
#14 2018-11-19 13:41:56
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
The use of Dry ice is a topic of and for use outside the living area where man is protected from the toxic levels of its use. So the exhaust exit could be a hose connection to a chamber for collection by the same night time freezing as I mention elsewhere as it will be clean and free of dust for reuse. On earth air tools operate over 40 psi to 120 range but on mars will we still need that same range for the co2 powered tools? The PSI range is used for torque and or for speed of movement where the cubic feet of volume is an important factor for how long the tool will be useable..
Offline
Like button can go here
#15 2018-11-19 20:25:14
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
International Journal of Greenhouse Gas Control
Volume 13, March 2013, Pages 26-33
Analysis of CO2 frost formation properties in cryogenic capture process
Abstract
With the development of CO2 capture technologies, the cryogenic approach provides a promising alternative for greenhouse effect mitigation. In previous work, a novel CO2 capture process was developed based on Stirling coolers (SC).
The results show that the frosted CO2 layer significantly influences the heat transfer between the gas stream and cooling fins. Over time (from 0 to 60 min), the thickness of the frost layer increased from 0 to 3.0 mm. The thermal conductivity increased from 0 to 0.4 W/(m K). When the flow rate was set at 1 L/min, the temperature of the cold head of the SC varied from −105.2 °C to −102.1 °C. When the flow rate was 3 L/min, the temperature rose from −106.3 °C to −98.0 °C.
Offline
Like button can go here
#16 2018-11-19 20:37:14
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
https://hal.archives-ouvertes.fr/hal-00718373/document
Design and simulation of a heat pump for simultaneous heating and cooling using HFC or CO2 as a working fluid
https://ntrs.nasa.gov/archive/nasa/casi … 009969.pdf
Frost growth and densification in laminar flow over flat ...
http://www.mars.asu.edu/christensen/cla … rus_82.pdf
Periodic Climate Change on Mars: Review of Evidence and ...
Offline
Like button can go here
#17 2018-11-20 17:47:52
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
How to Convert the Volume of Co2 Gas to Liquid
Converting between gas and liquid, therefore, depends entirely on pressure. For this calculation, convert between gas at one atmosphere of pressure around the freezing point of water, and liquid at room temperature and 56 atmospheres of pressure.
Determine the mass of the CO2 gas. At one atmosphere and 0 degrees C, the density of CO2 gas is 1.977 grams per liter. Multiply the volume, in liters, by 1.977 to get the number of grams of CO2. As an example, consider 1,000 liters of CO2 gas. Under these conditions, it would have a mass of 1977 grams, or 1.977 kilograms.
Divide the mass by the density of liquid CO2. Liquid CO2 at room temperature and 56 atmospheres is 0.770 grams per milliliter. In the example, this calculation would give a result of 2,567.5 milliliters. Convert units so that they can be compared. Divide milliliters by 1,000 to get liters for a comparison with the gas phase. In the example, the result is 2.5675 liters of liquid from 1,000 liters of gas.
here is video Orbiter Spots Carbon Dioxide Avalanche On Mars
So rather than mining the poles we should target these locations for the goal of co2.
Mars Orbiter Spots a Huge Avalanche of Carbon Dioxide on the Red Planet

The avalanche, which measures about 65 feet (20 meters) across, was spotted on a scarp at the edge of the North Polar layered deposits of Mars.
Offline
Like button can go here
#18 2018-12-05 13:03:55
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
Much like on Earth we will have areas that seem to get more solar energy than others even at the same latitude.
https://www.nrel.gov/gis/solar.html
Of course there will also be areas that will be colder and with higher concentrations of co2 for the purpose of getting it.
Why we see less energy as we transit farther away from the sun
https://www.geog.ucsb.edu/ideas/Insolation.html
Why its differrent with latitude
http://sites.gsu.edu/geog1112/solar-radiation-seasons/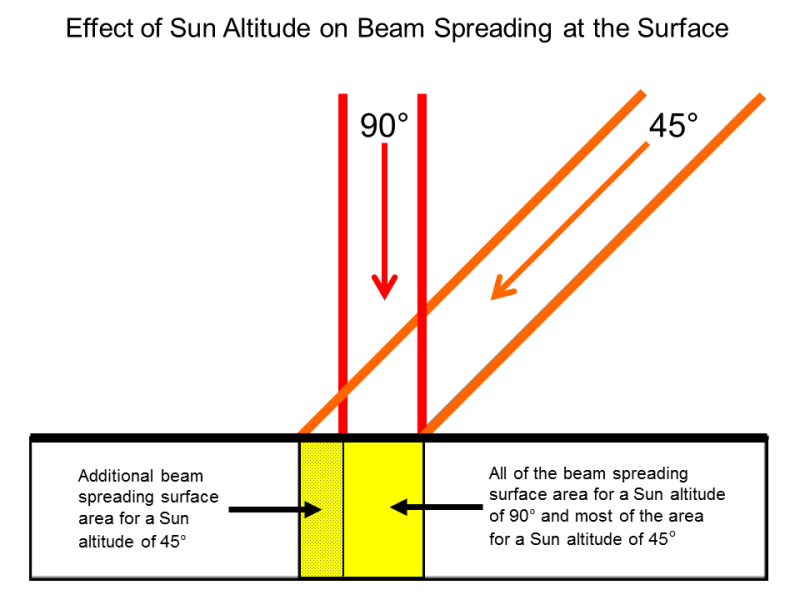
https://eesc.columbia.edu/courses/ees/c … index.html

time of day to season
Of course all of this is for working during the day from solar providing the co2 expansion of the collected dry ice to make the tools work.
Offline
Like button can go here
#19 2018-12-20 20:49:22
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
The trouble with CO2 for mars is just how thin it is and how much energy is required to get it to a useful pressure and quantity
We know that we can get dry ice with a slight nugde during the night from a cryo chiller plate of chamber but is still a small quanty of dry ice and is dependant on how larget the surface area is.
Then it needs to be moved before day light to a chamber to allow solar heating to rise the pressure in this chamber to force it to that next level.
So could a solar chimney help to create that rise in pressure for making it easier to compres Add to that concentrating panels to force the heat level up makes this as good as it can be without using tons of power.
https://www.math.purdue.edu/~lucier/The … himney.pdf
https://www.designingbuildings.co.uk/wiki/Solar_chimney
Offline
Like button can go here
#20 2020-01-05 16:51:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
bump to go with other topic where co atmosphere is imprtant to capture, filter and compress with the least amount of expended energy as possible.
RobertDyck's freezer is one of many methods that we could use.
NASA SBIR - 1998 (abstract only): Mars Atmospheric Carbon Dioxide Freezer
NASA Technical Report Server (NTRS) - 2012: Mars In Situ Resource Utilization Technology Evaluation
We have examined the technologies required to enable Mars In-Situ Resource Utilization
(ISRU) because our understanding of Mars resources has changed significantly in the last
five years as a result of recent robotic missions to the red planet. Two major developments,
(1) confirmation of the presence of near-surface water in the form of ice in very large
amounts at high latitudes by the Phoenix Lander and (2) the likely existence of water at
lower latitudes in the form of hydrates or ice in the top one meter of the regolith, have the
potential to change ISRU technology selection. A brief technology assessment was performed
for the most promising Mars atmospheric gas processing techniques: Reverse Water Gas
Shift (RWGS) and Methanation (aka Sabatier), as well as an overview of soil processing
technology to extract water from Martian soil.NASA NTRS - 2011: Evaluation of Mars CO2 Capture and Gas Separation Technologies
NASA NTRS - 2017 (slides): The Technology and Future of In-Situ Resource Utilization (ISRU)
This is the webview https://view.officeapps.live.com/op/vie … esting.doc
http://canada.marssociety.org/winnipeg/ … esting.doc
The freezing of co2 has been meantioned before in
http://newmars.com/forums/viewtopic.php?id=6826&p=12 at post 278
http://newmars.com/forums/viewtopic.php?id=7316&p=2 at post 36
Offline
Like button can go here
#21 2020-01-07 22:40:20
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
For mars we are trying to run the laws backwards for Expanding Gas: Boyle’s Law
So lets start with something that we have here on earth like a Co2 cartridge and see for what given size the volume that drops the pressure down to what we would see on mars. The small cartridgers are used for bike tire inflation here on earth but it will work to figure out the terms we need on mars.
https://reviews.mtbr.com/inflation-char … -co2-sizes
Offline
Like button can go here
#22 2020-01-08 17:51:53
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
The conversion of air which can be processed for the co2 there is an energy required to process a given volume at the parts per million rate with in the volume of air process which will give us a net mass of co2 at gas level and then at a liquid volume pressure for temperature.
That said how do we compute some of this?
How much volume does 1 kg of CO2 occupy at room temperature and standard pressure?
CO2 has a molecular weight of 44 g/mol
1 kg CO2 = 1000 g × (1 mol/44 g) = 22.7 mol CO2
V=nRT/P, V=(22.7)(0.0821)(300)/1 = 559 L CO2 at 27°C (300K), 1 atm
This is a little more than half a cubic meter approximately equal to the volume of two bathtubs or the trunk of a large car.
Offline
Like button can go here
#23 2020-08-30 07:49:11
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool

http://marsforthemany.com/wp-content/up … hPaper.pdf
The Feasibility of a Solid Carbon Dioxide Pressure Engine as an Energy Source for Mars Exploration and Colonization
Offline
Like button can go here
#24 2020-08-30 10:30:48
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,236
Re: Dry ice pneumatic tool
For SpaceNut re #23
Thanks for bringing this topic back to light.
I've added three vendors to PlotMaster (note that Plot0053 is a prime contractor for dry ice supply and services)
0107 Dry ice pneumatic equipment for stationary or mobile use Vendor #1
0108 Dry ice pneumatic equipment for stationary or mobile use Vendor #2
0109 Dry ice pneumatic equipment for stationary or mobile use Vendor #3
***
The student project you showed us is (or could be) the starting point for development of equipment for use on Mars.
It is conceivable that a system might be practical on Earth. Dry ice represents a form of stored energy similar to coal, in this respect ...
Coal needs a supply of oxidizer to produce useful output in the form of heat. That heat must be harnessed before it can be used.
Dry ice needs a supply of heat to produce useful output in the form of gas under pressure.
Assuming dry ice is manufactured, it represents the energy invested by the manufacturer.
On Mars, if dry ice is brought down from the poles, then the investment of energy needed for the transport is what the customer is paying for.
The Universe takes care of drawing heat energy out of CO2 to make dry ice on Mars.
Asking Google for the price of dry ice in the US gave this:
Dry ice is generally priced by weight, but the exact cost varies from one retailer to the next. On average, the price ranges between $1.00 to $3.00 per pound.
Dry Ice FAQs | All About Dry Ice: Cost, Quantities, & More ...
By drawing heat from the environment at room temperature, the student project was able to produce a certain amount of useful work per pound of dry ice.
You may well have computed what that amount was in your post(s) above, but if you are willing, it would definitely be interesting to see what could be obtained from a simple mechanical system using dry ice as the "fuel" and the thermal energy of the Earth atmosphere as the "oxidizer".
$1.00 (US) to $3.00 (US) per pound will produce X amount of work (ie, lifting water out of a well).
Meanwhile, cost of gasoline in the US varies by location but here is an average price per gallon for the US yesterday per Google: $2.234
Again per Google:
The Answer:
According to the Science and Technology Desk Reference, the weight of a gallon of common fuel (such as gasoline) is six pounds. A gallon of water, on the other hand, weighs about 8.4 pounds.Feb 23, 2017The Weight of Gasoline - Fact Monster
So (using those two figures) the price per pound (on average) in the US yesterday was $.39
It would seem that on a straight comparison of cost gasoline would be superior. However, the dry ice system produces no NEW CO2, while the gasoline solutions does. Furthermore, the gasoline solution requires a global infrastructure, which will not be present on Mars for some time.
If you (or anyone) were designing a reliable power delivery system for Alaska or any similar location at the poles of Earth, a dry ice solution might seem attractive.
The amount of supplies needed from elsewhere on Earth would (presumably) be limited to lubricant for moving parts and perhaps a replacement component now and then.
(th)
Offline
Like button can go here
#25 2020-12-16 11:10:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,826
Re: Dry ice pneumatic tool
bump
The business tools for mars will include many off the shelf uses to repair the equipment we bring to mars that will include some battery operated devices but with that we can also use many more that are not hand powered but by using the excess compressed or phase transitioned co2 to drive them with. Co2 gathering from the regolith frost layers as well as from the atmosphere are key to making it a dream come true. This would include the polar location mining and of course and glaciers that contain the dry ice with in them which is what is believed to be the case when they are dirt covered. With the bonus of possible water ice mixed in.
Offline
Like button can go here