New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2018-07-11 21:56:29
Units of Pressure
I have always been a strong proponent of SI units, and I think we should use them in all fields of life: I'd be glad to use kelvin to measure weather, meters for height, kilos for weight, meters per second for driving speed etc.
US units are annoying in general, but it occurred to me that there's an absolutely insane number of units for pressure. Consider the following list:
Pascals (Newtons/m^2)
Bars (100,000 Pa)--Millibars are often used also
Atmospheres (100,000 Pa or 101,325 Pa or sometimes 750 Torr depending who you ask)
Psi (lbf/in^2)
Psf (lbf/ft^2)
FSW (Feet of seawater; roughly 3064 Pa)
mmHg (Millimeters of Mercury; roughly 133 Pa)
inHg (Inches of Mercury; roughly 3386 Pa)
Torr (Roughly the same as a mmHg although not precisely)
mmH2O (Definitions vary based on reference temperature; Roughly 9.8 Pa)
InH2O (Definitions vary based on reference temperature; Roughly 249 Pa)
And then you could get plenty of other units by deriving backwards from other non-standard units like the calorie, BTU, or erg. For example, you might end up with a horrific frankenstein of a unit like BTUs per inch per acre.
These kinds of things can be frustrating because they make it hard to compare different measurements of the same or similar things. Just for example, today I tried to compare a vacuum (measured at 15 millitorr) to a high pressure gas (measured at 3504 psi) at a time when I didn't have a calculator on me. After a bit of arithmetic I figured it out (one is 12,000,000 times higher than the other) but it would have been much easier if they were shown as 24 MPa and 2 Pa.
Anyway, what gives? And what other annoying units have you all ran into?
-Josh
Offline
Like button can go here
#2 2018-07-12 04:39:22
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Units of Pressure
There are various sets of consistent units such as CGS, MKS and some imperial sets, each with a large number of derived units. It is perfectly possible to use slugs, feet and seconds as a basis. The main problems are shifting from one set to another and using non standard units such as bars in place of Pascals. Both of these involve conversions of the numbers and are a rich source of errors.
Offline
Like button can go here
#3 2018-07-12 06:59:27
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Units of Pressure
US ton, metric ton (tonne) and the British or Imperial ton...they are annoying because they are all quite similar in weight.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#4 2018-07-12 08:59:09
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,126
- Website
Re: Units of Pressure
I find that metric has just as many units idiocies as US customary. For example, the Germans use m-kgf for torque. That puts a kg-force unit out there which is not the same as a kg of mass. How is that idiocy different in any way from the idiocy of lbf vs lbm in US customary, other than a different number? You simply must understand the units you are using, and if they are not consistent, then don't forget the gc units conversion factor in F= ma: F = m a / gc. It's not hard, but you CANNOT be careless.
Point is, most of the metric world out there does not conform to SI-metric. Only the US promotes SI-metric only, and then doesn't actually use it. What I see in recent years is a mix of US customary and metric units and items being used widely in the US. This is really apparent under the hood of a car. You had better have full sets of metric and US customary tools if you attempt any work there. I do like the better quality standards used machining metric bolts: they do unscrew far easier once the fastening torque is loosened.
As for pressure, the common thread here is "atmospheres", which is not an SI unit. By "atmosphere", I mean sea level standard pressure, per the US and ICAO standard atmosphere models. These are 14.696 psia = 1 atm = 101.325 KPa. A "bar" is not quite the same as an atmosphere, being about 1% off: 1 bar = 100,000 Pa, when 1 atm = 101,325 Pa.
If you are using the calibrated altimeter in an airplane, it depends upon whether the mercury column height is measured in inches or mm. But one standard atm = 29.92 in Hg = 760 mm Hg. Easy enough. You just cope with what is customary (and what all your gear is calibrated in). Simple as that. When all else fails, report atmospheres.
There is real method to that madness of using atmospheres as a common basis. Consider the chemical composition of air, where the volume percentage is also the partial pressure percentage. If the oxygen content is 20.94% = 0.2094, the partial pressure of oxygen is numerically equal at 0.2094 atmosphere. From there, you can express it any damned way you want for working with the equipment you have on hand.
Tons were defined for convenience by various entities long before there was a metric or any other system, mostly in shipping and in agriculture. I'm surprised the various tons are as close as they are. It's just a legacy from history. Deal with it. Same for gallons.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#5 2018-07-12 17:38:39
- IanM
- Member
- From: Chicago
- Registered: 2015-12-14
- Posts: 276
Re: Units of Pressure
I agree with the difficulty of units for pressure. As a geologist I tend to intuit the best in atmospheres (and to an extent bars for the same reason) and take a while with stuff like psi or even Pascals. (On another note, I'm not the biggest fan of Celsius, intuiting in Fahrenheit and doing scientific work in Kelvin.)
As for history, the main advantage of imperial and other customary units is that many of their numbers divide evenly by more integers. An example is 12 inches in a foot dividing evenly into twos, threes, fours, and sixes, making it more convenient in a pre-modern society. Indeed, 5280 feet in a mile divide into every integer from 1 through 12 except for 7 and 9, as well as 15 and 16. It's also notable that the US probably would have been the second ever country to adopt the metric system (indeed, the US Dollar was the second ever decimal currency in the western world after the Ruble) were it not for souring relations with France and warming relations with Britain in the late 1790s. Oh well.
The Earth is the cradle of the mind, but one cannot live in a cradle forever. -Paraphrased from Tsiolkovsky
Offline
Like button can go here
#6 2018-07-12 17:44:50
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Units of Pressure
Comfortable with either unit types so long as they are clearly marked but for pressure it is PSI as this is common for car tires, basketballs and other type stuff that is inflated.
So long as its not a calculus equation I am fine......
Offline
Like button can go here
#7 2018-07-12 22:09:29
Re: Units of Pressure
One thing about different units that can get overlooked is that it's not always so simple as converting them to get the number you want.
Let's say you have an object that has a diameter specified as 0.250". If no spec is given for the acceptable variation, the implied tolerance is 0.250±0.0005". 0.0005" on 0.250" is a tolerance of ±0.2%.
Now, you might convert this to millimeters. 0.250" is 1/4 of an inch, which corresponds to 6.35 mm. If presented without acceptable variation the implied tolerance is ±0.005 mm, which is ±0.07%.
You might say that's no big deal--0.07% is less than 0.2%, after all. But tighter tolerances mean higher costs to manufacture. But it is different and it's something you need to be aware of. Plus potentially another number you need to append after you convert to SI units.
That's why it's best to stick to SI units the whole time, right from the start.
-Josh
Offline
Like button can go here
#8 2018-07-13 14:40:51
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,126
- Website
Re: Units of Pressure
The baseline standard practice for drawings is to explicitly show tolerances. There are two ways to do this. There is no excuse not to.
GW
Last edited by GW Johnson (2018-07-13 14:41:11)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#9 2018-07-14 15:59:34
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Units of Pressure
Volume of the item which has the pressure within can also alter the acuracy of the pressure as the walls of the item stretch depending on the materials.
Offline
Like button can go here
#10 2020-09-06 08:14:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Units of Pressure
bump units of measurement
Interesting weather charts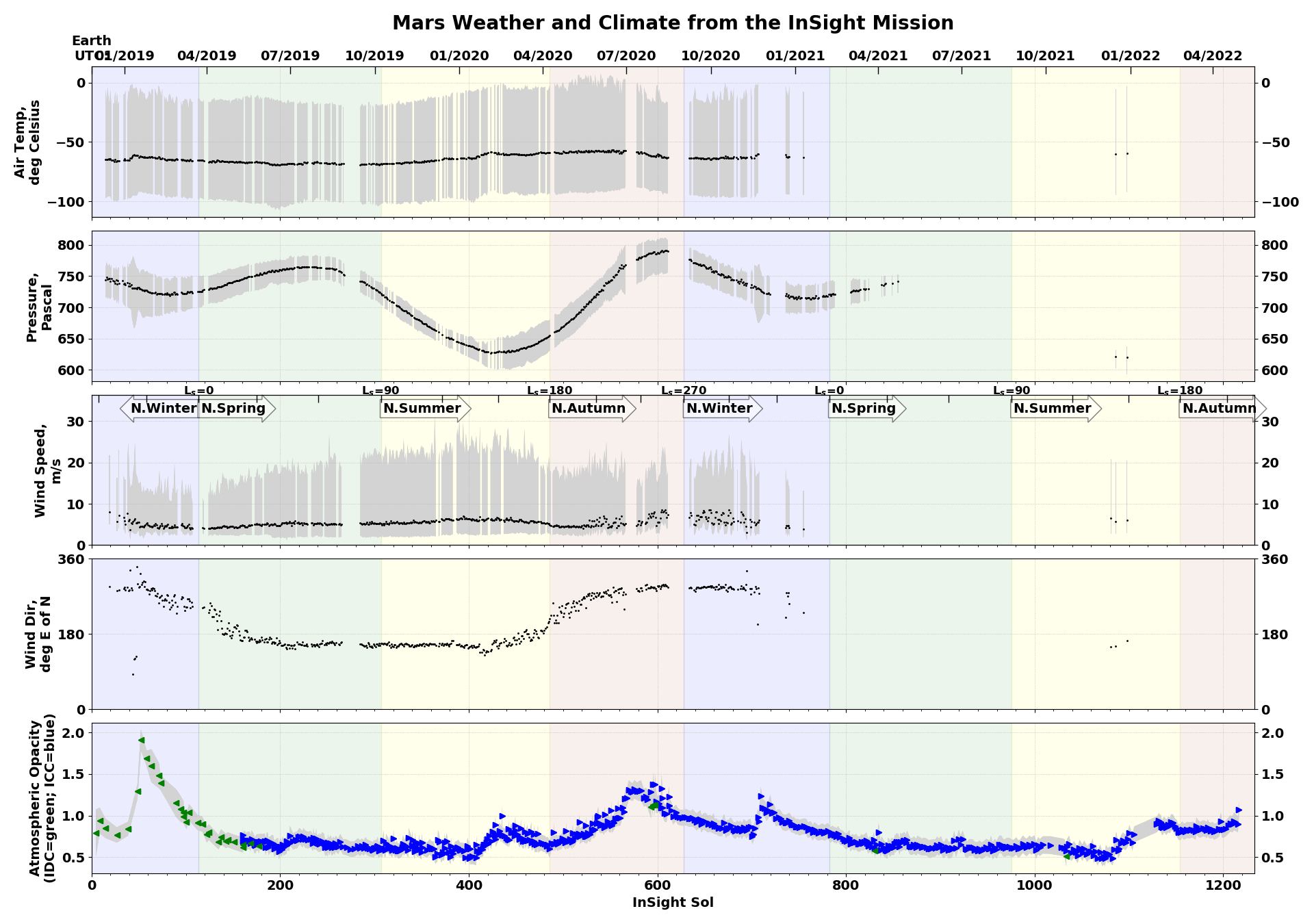
which shows summer with the increasing air pressure and dust increasing lagging for the seasonal change coming soon...
Offline
Like button can go here