New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2017-10-18 06:54:18
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Mars Atmospheric Kinetic Engine
A recent post on Martian compressed air energy storage got me thinking about a possible Martian renewable energy source that has thus far been overlooked. I have not yet performed any sort of detailed analysis of this yet.
https://commons.wikimedia.org/wiki/File … iagram.svg
The idea works like this:
1. Build a compressor station at the top of a mountain. Average Martian temperatures are very close to the triple point of CO2 (217K). This means that with intercooling, very little mechanical work would be needed to compress CO2 into a saturated liquid at typical Martian temperatures.
2. Run the liquid CO2 down the side of the mountain through a steel pipe.
3. At the bottom of the mountain, build a two stage turbine – the first would extract kinetic energy from the falling liquid CO2 much like a hydropower plant here on Earth; the second would boil the CO2 using either ambient heat or stored solar heat and extract thermodynamic work.
Provided the thermodynamic expansion stage provides sufficient energy to cover the energy of compression, kinetic energy of the falling CO2 would result in net energy generation. The idea is sort of like hydropower, but using liquefied CO2 as the working fluid rather than water.
Last edited by Antius (2017-10-18 06:54:45)
Offline
Like button can go here
#2 2017-10-18 07:09:30
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mars Atmospheric Kinetic Engine
That's a clever idea. You should patent it - quick! lol
Sounds feasible...as long as carbon dioxide is close to the liquid point...what is an efficient "drop"? I'm guessing it has to be something like 400 feet to work well.
Does moving downhill impart heat to the CO2 - would that be a problem?
A recent post on Martian compressed air energy storage got me thinking about a possible Martian renewable energy source that has thus far been overlooked. I have not yet performed any sort of detailed analysis of this yet.
https://commons.wikimedia.org/wiki/File … iagram.svgThe idea works like this:
1. Build a compressor station at the top of a mountain. Average Martian temperatures are very close to the triple point of CO2 (217K). This means that with intercooling, very little mechanical work would be needed to compress CO2 into a saturated liquid at typical Martian temperatures.
2. Run the liquid CO2 down the side of the mountain through a steel pipe.
3. At the bottom of the mountain, build a two stage turbine – the first would extract kinetic energy from the falling liquid CO2 much like a hydropower plant here on Earth; the second would boil the CO2 using either ambient heat or stored solar heat and extract thermodynamic work.Provided the thermodynamic expansion stage provides sufficient energy to cover the energy of compression, kinetic energy of the falling CO2 would result in net energy generation. The idea is sort of like hydropower, but using liquefied CO2 as the working fluid rather than water.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#3 2017-10-18 09:18:27
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Re: Mars Atmospheric Kinetic Engine
I am not sure about minimum feasible drop. I don't think heat addition will be a problem, in fact looking at the CO2 phase diagram I would say that for drop heights greater than about 1km, some heating is necessary to prevent the formation of dry ice under static or dynamic pressure. The pipes could conceivably clog up with dry ice if the system is left static for too long.
I know that such a system is unlikely to work on Earth. To liquefy air, one must extract approximately 300KJ/Kg of heat at a coefficient of performance of about 0.33 at 90K. So one must invest nearly 1MJ/kg to liquefy the air, whereas the gravitational potential energy for a drop of 2km, say, would be 20KJ/kg. Even if the device produces net energy, the EROI would be terrible, which means the economics would be terrible.
The unique properties of the Martian atmosphere, i.e. a gas close to its triple point temperature, means that this might be possible on Mars with a much lower embodied energy.
Offline
Like button can go here
#4 2017-10-18 09:48:04
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 4,000
- Website
Re: Mars Atmospheric Kinetic Engine
Maybe not for air, but if the air regularly gets close to it's dew point...
Use what is abundant and build to last
Offline
Like button can go here
#5 2017-10-18 10:54:06
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Mars Atmospheric Kinetic Engine
Condensing water vapour at high altitude is called a cloud. Clouds can be caught, as is done by the vegetation on the peak of Ascension Island. This is the source of virtually all the Island's moisture and sustains its (limited) agriculture. I don't think they have tried to get power from it as there would be more reliable sources.
On bigger mountains hydro power becomes practical, but generally one waits for the clouds to become rain to supply water in large quantities.
Offline
Like button can go here
#6 2017-10-18 20:13:02
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
I remember posting a simular thought less the RTG some where in one of the topics but it may have been lost in the great crash...
http://cr4.globalspec.com/thread/84740/ … -Generator
https://www.greenoptimistic.com/quasitu … effizVIrGg
http://solarhydrogensystem.com/the-system/
http://www.rexresearch.com/minto/minto.htm
https://www.motherearthnews.com/green-h … az76mazhar
http://www.redrok.com/engine.htm
http://www.popularmechanics.com/science … 0/4232571/
https://www.geothermal-energy.org/pdf/I … _Final.pdf

Offline
Like button can go here
#7 2017-10-18 20:47:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
http://www.scielo.org.co/pdf/dyna/v81n1 … 185a21.pdf
Over the last decade, organic Rankine cycles (ORCs) have widely been studied to harness low-grade heat sources that provide temperatures ranging between 80 and 400°C.
An ORC includes the same components as a traditional Rankine cycle, namely a pump, an evaporator, a turbine (or expander) and a condenser. The major difference comes from the choice of working fluid: water is replaced by an organic component.
Different technologies of concentrating collectors can be used for ORC solar power plants, such as solar towers, parabolic troughs, Fresnel linear collectors and solar dishes.
The liquid fluid is pressurized by a pump and vaporized to a superheated state by means of a heat input. The vapor is then expanded in a turbine connected to a generator. Finally, the vapor is condensed and heat is released into the environment. A regenerator is included to use the residual high-temperature vapor refrigerant exiting the expander to preheat the liquid refrigerant after the pump.
Offline
Like button can go here
#8 2017-10-19 10:04:58
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Mars Atmospheric Kinetic Engine
Working fluid doesn't have to be organic. For low temperature heat sources ammonia can be used. For very high temperature heat sources mercury. In the latter the mercury is condensed in a water boiler which is used as a second stage.
Offline
Like button can go here
#9 2017-10-19 10:14:08
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mars Atmospheric Kinetic Engine
I've wondered before now about regolith: if you had enough loose regolith on a suitable very high mountain could you use it to drive a turbine (simply pushed into a feeder hopper, using a bulldozer). Of course, eventually you run out of loose regolith, but then you can just move to the next mountain...land is not really an issue on Mars. ![]() Would probably be more like a traditional waterwheel than a modern hydro turbine I imagine.
Would probably be more like a traditional waterwheel than a modern hydro turbine I imagine.
However, I think the liquid CO2 powered turbine sounds good and definitely worth investigating.
Working fluid doesn't have to be organic. For low temperature heat sources ammonia can be used. For very high temperature heat sources mercury. In the latter the mercury is condensed in a water boiler which is used as a second stage.
Last edited by louis (2017-10-19 10:16:19)
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#10 2017-10-19 20:33:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
Power Cycles for Electricity Generation
Most of our development to this point has been oriented toward obtaining heated fluid from a solar collector. Often, the industrial demand to be satisfied by a solar energy system is for this heat. However, a more valuable form of energymechanical or electrical energy (both are equivalent in the thermodynamic sense)is sometimes desired either exclusively or in combination with thermal energy. The device used to produce mechanical work or electricity from solar generated heat is a power conversion cycle, or heat engine.
Several considerations peculiar to solar energy systems affect the choice of the power conversion cycle and how the solar energy system is designed to incorporate it. These considerations are discussed in this chapter along with a detailed discussion of the three power cycles usually considered for solar applications: the Rankine, Stirling, and Brayton cycles.
This development will follow the outline below:
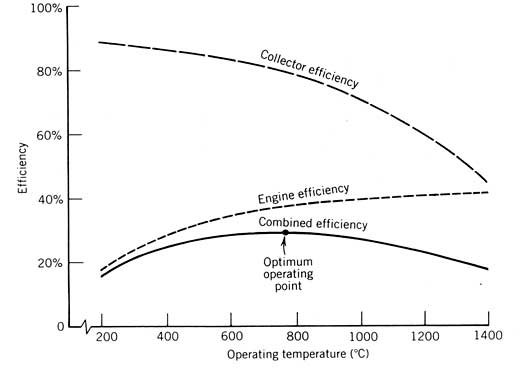
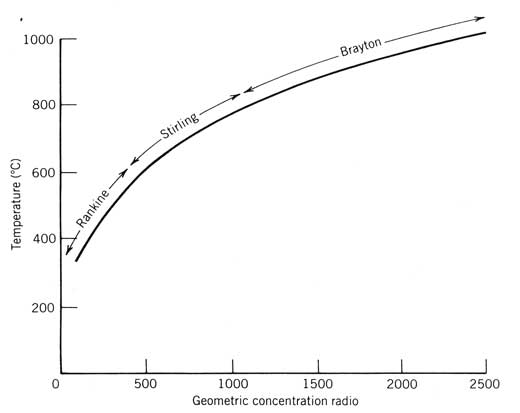
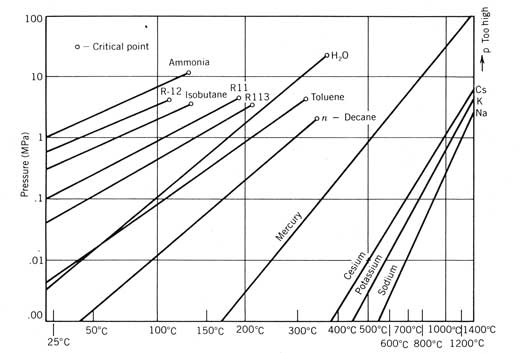
Experimental Study and Modeling of a Low Temperature Rankine Cycle for Small Scale Cogeneration
Offline
Like button can go here
#11 2017-10-20 08:39:58
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Re: Mars Atmospheric Kinetic Engine
I ran a few quick and rough EROI calculations on a Mars built solar dynamic system and was pleasantly surprised by the results.
Anhydrous liquid ammonia has good compatibility with carbon steel:
https://www.calpaclab.com/carbon-steel- … ity-chart/
It is also liquid across a temperature range of -75 to 25°C, the latter requiring a vapour pressure of 1MPa (10bar).
https://en.wikipedia.org/wiki/Ammonia_(data_page)
On this basis, I would propose that anhydrous ammonia would be an excellent working fluid for a solar or geothermal power system on Mars.
A solar thermal collector could be a flat clay plate (no cover glass needed in the vacuum of Mars) with low carbon steel tubes embedded in it, containing liquid anhydrous ammonia. During the day, the collector would collect heat at say 0°C. The cold store would be a tank of saline solution that will melt at -20°C, serving as a heat sink. At night, the cycle can be reversed. The heat sink becomes a heat source at -20°C and the panel will dump heat into space at a temperature of -70°C. At zero Celsius, ammonia boils at 4.3bar. At -20C, it boils at 1.9bar. Could we use a single stage LP turbine? It would have a feed pressure of 4.3bar during the day and back pressure of 1.9bar; and a feed pressure of 1.9bar at night and back pressure of 0.1bar. I don't honestly know. Assuming the device works at 2/3rd Carnot efficiency, the average efficiency of the device would be 9.5%.
If the steel tubes are 1cm in diameter and the steel has a yield strength of 250MPa, then a design factor of 5 would give a tube wall thickness of 0.1mm. If the tubes are spaced 5cm apart, then a total of 20m of tubing would be needed for 1m2 of panel. That would weigh in at 0.5kg steel per m2 of panel, not including manifolds or other pipework. If we roughly double steel mass to include those items and valves, we would need on average about 1kg of steel per m2 of solar plant. Let us assume an embodied energy of 30MJ/kg of new steel. The clay panels would have relatively low embodied energy. The thermal storage tank could simply be a pit, lined with polyethylene and filled with brine. The heat exchanger within the brine would probably consist of polyethylene tubes as these have a relatively low glass transition temperature, will not be corroded by the brine and can be manufactured from Mars-made ethylene gas.
Martian insolation is about 400W/m2 year-round average, close to the equator. If the system runs for 20 years at 9.5% efficiency, then each m2 of solar collector would produce some 8GJ of mechanical / electrical power over the course of its lifetime.
Based upon an energy investment of 30MJ/m2, the EROI of the panels would appear to be ~266. Of course, I have made a lot of simplifications here. I haven't included the energy required to build the thermodynamic plant, the salt water storage pit, or to mould and bake the clay panels with the tubes embedded in them. I have also assumed that the collectors are 100% efficient, whereas in reality, they will probably make half that. But even if EROI is an order of magnitude lower than I have estimated, an EROI of 26 is not bad for a round the clock power supply. As ammonia has good or excellent compatibility with mild and low carbon steels, it should be possible to cast, machine or 3D print most of the components on Mars.
As an aside, this sort of simple thermodynamic system would never be possible on Earth because:
1. Earth has insufficient diurnal temperature ranges for it to be an efficient option and simple brine-based phase change materials would not be useful;
2. Due to the thick atmosphere, Earth-based panels require evacuated glass tubes to build up decent temperatures, which ramps up the required energy investment dramatically;
3. Aside from deserts, Earth based northern locations where most people live do not benefit from persistent periods of direct sunlight. With little cloud cover, Mars would have clear sky most of the time;
4. Earth based panels must be insulated to prevent heat losses to the surrounding air and surroundings due to radiation. Mars based panels will lose no heat to surrounding air and only half as much to radiation; because their operating temperature in full sun will be close to 0°C rather than 50°C.
This is why on Earth; solar power tends to rely on PV. This has high embodied energy and poor EROI, especially when storage is factored in. All in all solar thermal power looks a lot more doable on Mars than it is on Earth. The system works well with low grade easily manufactured materials: baked clay, polyethylene sheeting and pipes and carbon steels. Energy storage is achieved prior to generation in the phase change of brines. These should be cheap on Mars, as water is available as ice and the soils are saturated with salts and chlorates.
Of course, one downside of an extended solar based power system is the large amount of EVA time needed to assemble and maintain the system on the surface of Mars. A 10MWe system would need to cover an area of about 1km2. But it would only need to be built once.
Last edited by Antius (2017-10-20 08:48:48)
Offline
Like button can go here
#12 2017-10-20 09:14:58
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Re: Mars Atmospheric Kinetic Engine
Darn! Silly me! At a temperature of -70C (200K) the system will only radiate 90W/m2 into the Martian night.
Earthenware clay is fired at a temperature of 760C.
https://www.goshen.edu/art/DeptPgs/rework.html
Assuming a clay density of 2000kg/m3, a 1m2 x 0.02m thick clay panel would weigh 40kg. If specific heat is 1KJ/KgK, then some 30.4MJ of heat would be needed to heat the clay to sinter temperature. So the embodied energy of the panel is 60.4MJ/m2. That reduces the panel EROI to about 33. Not as good as I originally thought, but still better than PV when storage losses are accounted for and much easier to make on Mars.
According to this link, a SKODA 660MW supercritical steam turbine has a weight of 1000te and an efficiency of 51%.
http://e2010.drustvo-termicara.com/reso … /fiala.pdf
That's 660W/kg. If the ammonia turbine has efficiency of 9.5%, then power density would be 123W/kg - if working fluid energy density and flow rates were the same, which of course they aren't. Since supercritical steam enters the turbine at a pressure of at least 22MPa, it would have roughly 50 times the energy density of ammonia vapour in our system. So the ammonia turbine would have at least an order of magnitude lower power density, although the lower pressure differential may allow thinner blades. If power density is ~10W/kg, then the turbine embodied energy (steel alone) would be about 16% that of the panels. All in all, a whole system EROI of 20 still looks achievable.
Last edited by Antius (2017-10-20 09:31:40)
Offline
Like button can go here
#13 2017-10-20 12:04:06
Re: Mars Atmospheric Kinetic Engine
Referencing my earlier posts on EROI, I don't think it's reasonable to say that speculative EROI calculations are anywhere near right or that they should carry much weight in our analyses.
-Josh
Offline
Like button can go here
#14 2017-10-20 12:24:21
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Re: Mars Atmospheric Kinetic Engine
Referencing my earlier posts on EROI, I don't think it's reasonable to say that speculative EROI calculations are anywhere near right or that they should carry much weight in our analyses.
I don't agree. My own efforts are not very robust. But a comprehensive analysis is a good proxy for telling us how the economics of a concept will pan out, if we exploit things like scale economies and if regulatory issues do not exist. EROI tells us how good something might be.
Offline
Like button can go here
#15 2017-10-20 12:57:40
Re: Mars Atmospheric Kinetic Engine
Well my point in the other thread was not so much that comprehensive EROIs are hard but that they are impossible, because there can be no consistent definition of EROI. At best, including labor, you will end up with a number that's almost precisely equal to a cost-benefit analysis
-Josh
Offline
Like button can go here
#16 2017-10-20 19:55:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
Back to the other parts of Antius post # 11 & 12, I take it that you are planning to create the flat clay plate (no cover glass needed in the vacuum of Mars) with low carbon steel tubes embedded in it, containing liquid anhydrous ammonia all from insitu materials and thats not bad of course less pumps. The flat plate clay collector could be covered by thermo blankets like those used in survival kits just place them on a roller system to cover the collector at night and wait until the sun hits it in the morning to uncover it.
Continuing with the rest of the system plan of a thermal storage tank could simply be a pit, lined with polyethylene and filled with brine will need an insulator to keep the ground from wicking away the hard earned heat and this holds true for the clay flat plate collector as it would have the same issue if making contact with the ground.
The heat exchanger filled with brine making use of polyethylene tubes makes for easy coiling of it within the pit will give plenty of hot water if we put it into another tank for the crew to shower with.
Adding a heat loop from the RTG to the pit is a plus for making it a continous output system.
Now back to the post #1 which when I reread it is describing an upside down, down draft chimney system where the opening at the top is greatly larger than the one at the bottom and with the help of an active cooling system gridding inside the unit we can liquify the CO2 from the power we are generating from the other system.
If fact this could be just a spiraling mining project to create the chimney from any hill or mountain creating that funnel effect for the condensing and force to aid in liquifing the co2 for later use.
Offline
Like button can go here
#17 2017-10-21 18:49:32
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Offline
Like button can go here
#18 2017-10-22 17:15:54
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
I have been looking at the types of pumps, compressors and turbines to make use of in the design; and common used parts that can do both as applied in the schematics of the system.
https://en.wikipedia.org/wiki/Axial_compressor

Of course the ends are capped and would have flow check valves to cause the correct rotation to happen and one would connect the shafts to a generator or alternator design to allow power to be created by the motion of the axial unit.
Offline
Like button can go here
#19 2017-10-29 06:58:33
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Mars Atmospheric Kinetic Engine
CO2 has been used as a refrigerant in the past- quite extensively. It has the drawbacks that pressures are very high and its critical point has quite a low temperature. When the heat sink gets a bit warm it can't be used in a regular reversed Rankine cycle refrigeration system. Apart from being flammable, propane is much more convenient.
Offline
Like button can go here
#20 2019-12-11 07:23:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
making the day night cycle work for man to gain co2
Offline
Like button can go here
#21 2020-03-02 20:22:29
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
This may be the older topic of a new one that worked on how do we build up power from sun light and captured co2 as its heated.
Offline
Like button can go here
#22 2020-03-03 06:57:34
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Mars Atmospheric Kinetic Engine
An interesting idea. The problem I can see is that the density of Martian air at ground level is something like 0.013kg/m3. That means a very large collector area would be needed to gather substantial amounts of gas.
The CO2 would not need to be condensed all the way down to liquid. At -60C and 1bar pressure, it would be much denser than air would be at sea level on Earth. If gas is injected at the top at 1 bar pressure, say, and the tube is long enough, then hydrostatic pressure would start to liquefy the CO2 at the bottom of the tube, without any additional compressor work. Some amount of compressor work is needed to take the CO2 from ambient pressure to injection pressure, at the top of the tube. The work needed should be modest as the CO2 is beneath its critical temperature of 31C.
If anyone has the time to hunt down detailed information on CO2 properties, they could work out how long the tube would need to be to accomplish this. It would require a bit of integration, because density would increase with increasing hydrostatic pressure, until it eventually reached liquid state.
The process would be most efficient when the atmosphere was at its lowest temperature, i.e. night time. We can either store night time cold and use solar power to compress the CO2 during the day, or we can store mechanical energy from solar power and compress the CO2 at night. I think the first is probably better, as heat (cold in this case) is much cheaper to store than mechanical energy.
Last edited by Calliban (2020-03-03 07:01:53)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#23 2020-03-03 10:35:41
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,305
Re: Mars Atmospheric Kinetic Engine
Apologies. I just reread the author's original post. He is discussing compressing atmospheric CO2 into a liquid at the top of a mountain, running it down the mountain through a pipe and building a hydroplant at the bottom to extract its kinetic energy. If the drop is big enough, then the energy harnessed from the falling liquid CO2 (at the bottom) would be sufficient to power the compression plant at the top of the mountain. Hence, no other power supply is needed and the liquid CO2 at the bottom is 'free' energy.
NIST provide a very useful tool that provides the thermodynamic properties for a variety of substances across a range of temperatures and pressures. Select carbon dioxide. Put all units into SI. Put the temperature at a constant 220K (-53C). Select starting pressure at 0.001MPa. Put final pressure at 0.75MPa. Select increments of 0.001MPa.
https://webbook.nist.gov/chemistry/fluid/
The CO2 will liquefy at a pressure of 0.599MPa (6bar). The enthalpy change of compressing the CO2 from 0.001MPa to 0.6MPa, is 12.4KJ/kg. This is the compressor work. The Cp of CO2 across the cycle averaged at 0.84KJ/Kg.K. So total temperature rise during compression will be small. However, the CO2 will need to dump some 345KJ/kg of latent heat in order to condense into liquid. This heat will need to be dumped into a radiator on top of the mountain. So the cycle would work better at night.
To provide 12.4KJ/Kg of compressor work using a hydropower plant at the bottom would require a drop of 3326m, assuming perfect efficiency. If the compressor at the top and turbine at the bottom are 80% efficient, the required drop increases to 5200m (16,900'). That is quite a large mountain. And the air will be thinner at the top making the compression job more difficult. The Martian atmosphere is so thin that a very large compressor inlet would be needed to gather the required CO2 for compression. Even though compressor work is small, the large rotating machine will suffer frictional losses that would make it relatively inefficient.
It might be easier to take a small proportion of the liquid CO2 and use stored heat to boil it, expanding the vapour through a turbine, which can then drive the compressor. That way, a tall mountain is not needed. The remaining liquid CO2 can be stored in a tank and will remain liquid at a pressure of 6bar at 220K. Energy can be recovered by boiling the CO2 using stored solar or nuclear heat (or even ambient heat) and expanding the vapour through a turbine.
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#24 2020-03-03 12:18:20
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mars Atmospheric Kinetic Engine
Couldn't we just mine frozen CO2 at the South Pole, and bring it to wherever our base was in the equatorial zone? Or does it need to be even more compressed?
And once you have your CO2 at the right pressure, is it a one-time application pretty much ie you are recycling it from liquid to gas?
An interesting idea. The problem I can see is that the density of Martian air at ground level is something like 0.013kg/m3. That means a very large collector area would be needed to gather substantial amounts of gas.
The CO2 would not need to be condensed all the way down to liquid. At -60C and 1bar pressure, it would be much denser than air would be at sea level on Earth. If gas is injected at the top at 1 bar pressure, say, and the tube is long enough, then hydrostatic pressure would start to liquefy the CO2 at the bottom of the tube, without any additional compressor work. Some amount of compressor work is needed to take the CO2 from ambient pressure to injection pressure, at the top of the tube. The work needed should be modest as the CO2 is beneath its critical temperature of 31C.
If anyone has the time to hunt down detailed information on CO2 properties, they could work out how long the tube would need to be to accomplish this. It would require a bit of integration, because density would increase with increasing hydrostatic pressure, until it eventually reached liquid state.
The process would be most efficient when the atmosphere was at its lowest temperature, i.e. night time. We can either store night time cold and use solar power to compress the CO2 during the day, or we can store mechanical energy from solar power and compress the CO2 at night. I think the first is probably better, as heat (cold in this case) is much cheaper to store than mechanical energy.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#25 2020-03-03 17:41:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,621
Re: Mars Atmospheric Kinetic Engine
That would require nuclear power levels in order to keep equipment and personnel from freezing leaving energy for the mining and processing of it for use elsewhere.
There is no need to have it roar to life on the first cycle as it can do a warm slow build up where the exhaust not going back into the atmosphere as it can be sent to a tank for later reuse until we gain enough co2 to make sure that it will have the capacity to run. Its the heat source that when its concentrated can make the low level of co2 work even harder.
Offline
Like button can go here