New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2017-01-14 12:42:47
- Void
- Member
- Registered: 2011-12-29
- Posts: 7,831
Ore resources on Mars
Spacenut. Here is a generic reference for the various items you have been stimulating.
I have read a bit of it and it suggests both metal and hydrocarbon resources might be found due to impact events.
https://en.wikipedia.org/wiki/Ore_resources_on_Mars
Example:
Quotes:
The surface of Mars contains abundant evidence of a wetter climate in the past along with ice frozen in the ground. NASA's Mars Odyssey actually measured the distribution of ice from orbit with a gamma ray spectrometer.[35] So, in the past, much water could have been available to circulate in cracks and deposit new minerals. This process, called hydrothermal alteration has been found in a meteorite from Mars. Research, published in February 2011, detailed the discovery of clay minerals, serpentine, and carbonate in the veins of a Nakhlite martian meteorite.[36][37] The Phoenix lander, whose rocket engine blast actually exposed a layer of ice, watched ice melt (the ice disappeared by sublimation).[38][39]
Because 30% of the roughly 180 impact craters on Earth contain minerals or oil and gas, it seems that the cratering promotes the development of natural resources [40]

Hellas Basin Area topography. This is one of the impacts that would have taken many thousands of years to cool. A great deal of minerals could have been deposited while this area was cooling.
A great prize would be a source of natural gas or even oil. Many will dispute that it could be on Mars. Some allow for abiotic natural gas. However we don't know if Mars was or is dead, so even a biotic source where an impact cooked out natural gas and oil, is not yet against possibility.
And I note that Hellas is attractive due to it's glaciers, and higher air pressure.
And I will beat this drum again Boom! Boom! Boom!
Quote:
Dark sand dunes are common on the surface of Mars. Their dark tone is due to the volcanic rock called basalt. The basalt dunes are believed to contain the minerals chromite, magnetite, and ilmenite.[48] Since the wind has gathered them together, they do not even have to be mined, merely scooped up.[49] These minerals could supply future colonists with chromium, iron, and titanium.
Dunes on the Rim of the Hellas Impact Basin
http://mars.jpl.nasa.gov/multimedia/ima … ageID=5697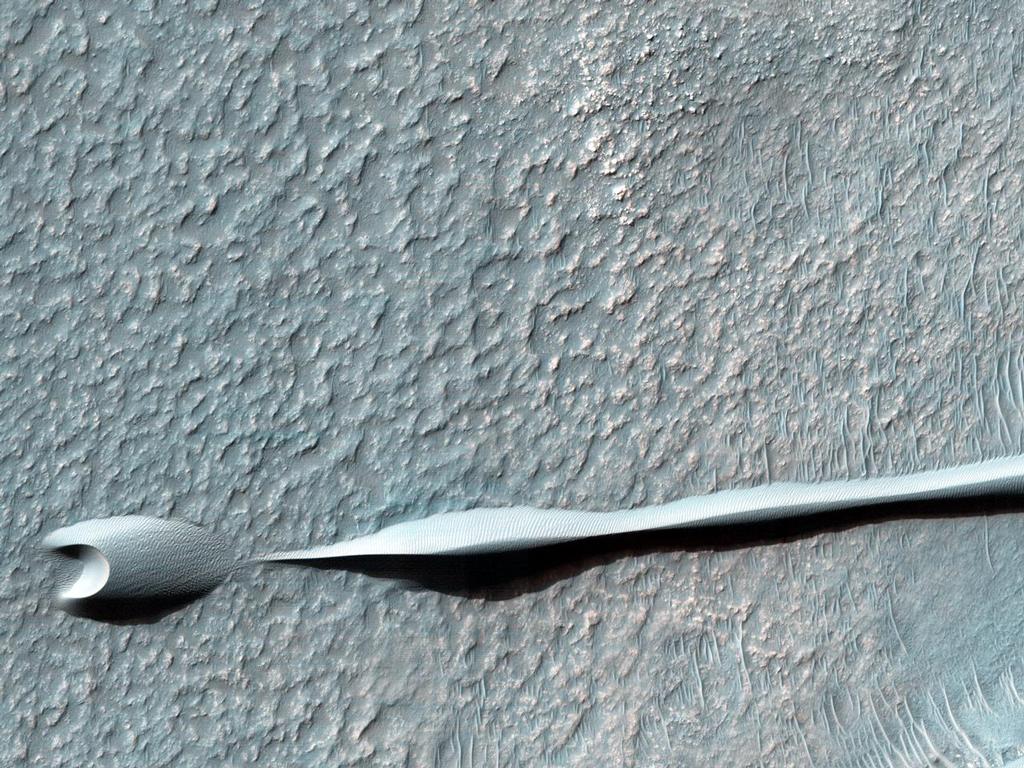
Oil on Mars? Debatable. Natural Gas? More likely.
http://oilonmars.blogspot.co.uk/
https://en.wikipedia.org/wiki/Abiogenic … eum_origin
Quote:
The abiogenic hypothesis regained support in 2009 when researchers at the Royal Institute of Technology (KTH) in Stockholm reported they believed they had proven that fossils from animals and plants are not necessary for crude oil and natural gas to be generated.[6][7]
https://www.youtube.com/watch?v=CyFj5ygqvd4
So at this time in my opinion we have a "He said/She said" situation as far as abiotic oil is concerned. But as I previously mentioned, if panspermia or another factor allowed life to exist on Mars previously then you can't discredit the potential for oil on Mars just by saying that abiotic hydrocarbons cannot exist on Mars.
The finding of Natural Gas on Mars would be incredibly useful.
Antius has indicated that there is even a potential to react Methane with CO2, which took me by surprise.
But beyond that if you reformed, Methane using solar power, and produced Hydrogen, you could certainly react that with the atmosphere of Mars, to produce a lot of things.
And in the process, you might manufacture Carbon Monoxide, which could be used in metallurgy.
For a local situation like Hellas, with the thin Martian atmosphere, transfer of bulk materials by mass driver, and ballistic travel to impact, may be possible. Antius first suggested this as I recall.
Done.
Last edited by Void (2017-01-14 13:10:31)
End ![]()
Offline
#2 2017-02-20 02:37:03
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
Quote:
Dark sand dunes are common on the surface of Mars. Their dark tone is due to the volcanic rock called basalt. The basalt dunes are believed to contain the minerals chromite, magnetite, and ilmenite.[48] Since the wind has gathered them together, they do not even have to be mined, merely scooped up.[49] These minerals could supply future colonists with chromium, iron, and titanium.
Mars is known for dust storms and dust devils. Can these fluxes of particles be collected, in any way?
Offline
#3 2017-02-20 11:17:55
- SpaceNut
- Administrator

- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,433
Re: Ore resources on Mars
Identifing that they resources are there is only part of the equation as we need the vehicles and equipment to gather it as well as to process it with ample energy resources to make it happen. These are the tall order for anyone launching vehicles to land on mars at this point.
Offline
#4 2017-02-21 03:43:52
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Ore resources on Mars
An electro magnet would pick out the magnetite particles from dune sand, which could be smelted with methane and purified as iron carbonyl. Maybe.
Offline
#5 2017-02-21 17:36:56
- SpaceNut
- Administrator

- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,433
Re: Ore resources on Mars
The dune sand will have more than just iron to remove as useful minerals and if you are going to move near it you might as well pick it up and fully process it....which would include processing out the water content as well as silica and anything else that is low hanging fruit from expending the energy to move it to be processed.
Offline
#6 2017-02-21 22:33:52
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
Fantasy again, wastes from the Earth that can help Mars readily are the red mud (metal oxides in alkaline) and phosphogypsum -- CaSO4 hydrate with heavy and radioactive elements.
By mixing them (?), light and heavy metal and radioactive element oxides are recovery with metal sulfates and water. Dried oxides and sulfates are crashed onto selected location on Mars in one giant rocket load. The radioactive element would be needed for nuclear reactors. The metals and sulfur provide readily some elements for early Martian settlers before the process of managing native Martian ores.
http://www.iaea.org/inis/collection/NCL … 056392.pdf
http://www.sciencedirect.com/science/ar … 9614001818
http://www.kuleuven.rare3.eu/wp-content … R_2014.pdf
In sum, the elements included are Nickel Copper Zinc Cadmium Chromium Titanium Manganese Samarium Neodymium Thulium Dysprosium Praseodymium Scandium Yttrium Erbium Ytterbium Gadolinium Lanthanum Cerium, not to mention the obvious ones: sulfur, aluminum, iron, calcium, sodium, uranium and thorium.
The difficulty is how to extract these elements from the residue that scattering on the crashing site.
Last edited by knightdepaix (2017-02-21 22:34:33)
Offline
#7 2017-02-21 23:41:00
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 7,936
- Website
Re: Ore resources on Mars
The books Red/Green/Blue Mars talk about a device that harvests Mars regolith and separates all the elements for useful construction material. However, in real life commercially mined ores have an extremely high concentration of just one material. Rarely two: the mineral ilmenite can be smelted for iron. The tailings can be smelted to produce titanium.
I have suggested mining hematite concretions as iron ore. At Meridiani Planum the concretions are embedded within jarosite, and other soft minerals. The concretions are hard, so can be separated by crushing, tumbling, and sifting. The pure concretions can then be crushed by a stronger rock crusher, then smelted for steel.
I've also said anorthite or bytownite can be processed to produce aluminum. Orbiters identified bytownite, but rovers found minerals are not exactly what orbiters expected. Besides, orbiters expected 25% bytownite; how do you separate that from regolith? Ideal is a highly concentrated deposit. And ideal is anorthite; bytownite has more albite. Plagioclase feldspar with too much albite will produce a film of quartz, which will seal the grain so acid cannot act at all.
Speaking of which, most bytownite is just a type of feldspar ore. However, some can form transparent crystals. Large crystals can be polished like gem stones. I wonder if Mars will have these? (Golden Sunstone from Mexico)
Last edited by RobertDyck (2017-04-25 02:19:03)
Offline
#8 2017-02-22 05:20:52
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Ore resources on Mars
One point I would make is that at least in terms of an early colony we don't want to be too bound by Earth economics. On Earth competing corporations try to produce pure metals etc at the cheapest cost, and that clearly means they like to work with the richest ores. But they are dealing in millions of tonnes of stuff...the early colony on Mars will only need perhaps hundreds of kgs or a few tonnes of metals each year to function well. It will have a surfeit of energy - so using energy to break down ores and/or collect material - will not be a big issue. And cost will not be a major factor in a multi-billion dollar project - the fact your metal refining process on Mars would multiply costs by ten or even a thousand on Earth will not prevent it being the best choice. In the early colony the big shortage, the most precious resource will be human labour/time.
The books Red/Green/Blue Mars talk about a device that harvests Mars regolith and separates all the elements for useful construction material. However, in real life commercially mined ores have an extremely high concentration of just one material. Rarely two: the mineral ilmenite can be smelted for iron. The tailings can be smelted to produce titanium.
I have suggested mining hematite concretions as iron ore. At Meridiani Planum the concretions are embedded within jarosite, and other soft minerals. The concretions are hard, so can be separated by crushing, tumbling, and sifting. The pure concretions can then be crushed by a stronger rock crusher, then smelted for steel.
I've also said anorthite or bytownite can be processed to produce aluminum. Oribers identified bytownite, but rovers found minerals are not exactly what orbiters expected. Besides, orbites expected 25% bytownite; how do you separate that from regolith? Ideal is a highly concentrated deposit. And ideal is anorthite; bytownite has more albite. Plagioclase feldspar with too much albite will produce a film of quartz, which will seal the grain so acid cannot act at all.
Speaking of which, most bytownite is just a type of feldspar ore. However, some can form transparent crystals. Large crystals can be polished like gem stones. I wonder if Mars will have these? (Golden Sunstone from Mexico)
https://upload.wikimedia.org/wikipedia/commons/thumb/a/a6/Bytownit-G-EmpireTheWorldOfGems.jpg/220px-Bytownit-G-EmpireTheWorldOfGems.jpg
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
#9 2017-02-22 09:52:29
- Oldfart1939
- Member
- Registered: 2016-11-26
- Posts: 2,452
Re: Ore resources on Mars
If we are using the apparatus sent to produce O2, the by-product is also CO, a "reagent" found in the Blast Furnace equations for manufacture of iron. It could also be used in production of carbonyls and purification of other metals. If we're producing metric ton quantities of LOX for the ERV, there will be even more CO produced. Why waste it?
I don't know enough about 3-D printing to suggest the use of metal carbonyls as "ink" in printing metallic objects.
Offline
#10 2017-02-22 11:05:14
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
In the early colony the big shortage, the most precious resource will be human labour/time.
https://phys.org/news/2017-02-metalloid … iming.html
RobertDyck may be delighted to hear that news. TeO3 2- are converted to Te.
http://www.sciencedirect.com/science/ar … 0505001174
The organic carbon is lactate. A nitrogen source can be ammonium chloride. Gaseous hydrogen and carbon dioxide can be used with lactate.
Metal oxides in Martian regolith are prevalent so in theory, a biotechnological factory where metals and metalloids are recovered from tanks of bacterial culture. Native organic carbon carbon, hydrogen and metal oxide ores are consumed to also produce byproduct carbon dioxide and water. Structural formula of lactate can be a combination of ethene, water and carbon dioxide. From RobertDyck, we know Zubrin claimed that ethene can be produced from Martian atmosphere and water, or is it? So in essence, two factories -- one bacterial biotech as mentioned, the other convert biotech byproduct and atmosphere carbon dioxide and water ice to make lactate and refine the metal and metalloids from the bacterial biotech factory.
Not to mention, the labor can be robotic at the bacterial biotech factory and automatic under supervision at the chemical factory.
Offline
#11 2017-02-22 11:42:49
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 7,936
- Website
Re: Ore resources on Mars
All iron ore on Earth is a form of iron oxide. The primary energy is to remove oxygen. This is not done with methane, it's done with either carbon monoxide (CO) or hydrogen gas (H2). Smelters on Earth burn coal in a furnace deliberately starved of oxygen to ensure as much as possible is converted to CO, not CO2. That hot CO then combines with iron oxide, stripping oxygen to form CO2. That leaves metallic iron. All coal dug from the ground has sulphur, and even a tiny bit of sulphur dramatically weakens steel. So coal is prepared by baking it and blowing air through to burn off sulphur without burning carbon. The result is a light sponge of carbon called "coke". That coke is then burned in an smelting furnace. Baking off sulphur produces yellow smoke, sulphur dioxide, which used to be released to the atmosphere. However sulphur dioxide combines with moisture in clouds and UV light from sunlight to produce sulphuric acid. When those clouds rained, that's acid rain. To prevent that, a "coking oven" that bakes coal to produce coke directs smoke through a shower of water with UV lamps. That produces sulphuric acid that can be sold for commercial uses. Producing steel in a blast furnace this way dissolves carbon into molten iron, producing steel so high in carbon that it's too hard and too brittle. Carbon used to be burned off by melting this steel in a Bessemer converter, blowing air through to burn off oxygen. Because you're deliberately adding oxygen, you have to be careful not to add so much that it oxidizes iron. The "direct iron method" instead adds hydrogen to the blast furnace. This also combines with oxygen from iron oxide ore, but produces water instead. Water doesn't remain dissolved in molten steel, so this adds less carbon to start with. Hydrogen is more expensive than coke, but it means you don't have to re-melt steel in a separate process. Overall, the direct iron method is less expensive; in terms of money or in energy. However, the direct iron method only works with high quality ore. The good news is hematite concretions are high quality ore.
The iron carbonyl method only works with metallic iron or steel (iron with carbon dissolved in it). It doesn't work with oxide ore. Iron meteorites are metallic, but terrestrial iron is all oxide.
Smelting steel on Mars will require carbon monoxide and hydrogen. Hydrogen come from electrolysis of water. CO2 is harvested from the atmosphere of Mars, there are no other sources of carbon that we've found on Mars. And we don't expect to find any coal. On Earth coal was formed when dead trees fell at a time before fungi evolved that can break down wood. Dead trees would just accumulate on the forest floor. Eventually dead trees would be buried deeply enough that pressure and heat would break down the wood to produce coal. Once fungi and bacteria evolved to break down wood, coal didn't form any more. Mars never had forests, so no coal. We can make CO on Mars by reacting CO2 with H2 over a catalyst. This combines some of the oxygen from CO2 to produce water. That water is recycled back into hydrogen via electrolysis. So everything on Mars always comes back to electrolysis of water.
Offline
#12 2017-02-22 12:30:57
- Oldfart1939
- Member
- Registered: 2016-11-26
- Posts: 2,452
Re: Ore resources on Mars
Robert-
My above post #9 simply pointed out that CO would be available in XS from the "Moxie" unit being proposed for the next Mars Rover which "may fly" in 2020. In order to use additional Hydrogen, a reclaimable source of water must be found for electrolysis first. Bringing LH2 from Earth would be inconceivable to me.
Offline
#13 2017-02-22 14:36:53
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 7,936
- Website
Re: Ore resources on Mars
The idea of bring LH2 from Earth was proposed only for the first science missions. It was proposed in 1990 when there was no confirmed sources of water at all. Even today we would have to do a lot of work before committing human lives to an in-situ water resource. But yes, local water is required for permanent settlement. You would never smelt with hydrogen brought from Earth.
Offline
#14 2017-02-22 17:10:55
- Oldfart1939
- Member
- Registered: 2016-11-26
- Posts: 2,452
Re: Ore resources on Mars
Robert-
Indeed; bringing LH2 from Earth was part of the Sabatier Reaction proposal by Robert Zubrin as one of the key points in Mars Direct. The entire concept of smelting Iron and manufacturing Steel will undoubtedly be in a second generation of missions directed towards true colonization.
Offline
#15 2017-02-23 05:17:46
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Ore resources on Mars
Meantime you could beat a bit of Nickel Iron asteroid into shape if you need a metal item. If the rover can find one, I'm sure a human can.
Offline
#16 2017-02-26 18:47:09
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
The idea of bring LH2 from Earth was proposed only for the first science missions. It was proposed in 1990 when there was no confirmed sources of water at all. Even today we would have to do a lot of work before committing human lives to an in-situ water resource. But yes, local water is required for permanent settlement. You would never smelt with hydrogen brought from Earth.
http://science.sciencemag.org/content/327/5963/313
Given the technological advance in carbon fixation, capture and storage, fixation carbon dioxide by electrosynthesis into oxalic acid is possible. Break down the oxalate into carbon monoxide and carbonate. One is a gas, another is an ion in solution. Then carbonate can be recycled for making more oxalate. For every two carbon used, one carbon monoxide is harvested. Adding electrolysis of water into consideration, in pure theory using ONLY in-situ Martian water and carbon dioxide resources are feasible to yield carbon monoxide and hydrogen.
Then accumulate enough carbon monoxide, applying the recipe in the Case of Mars to turn CO into carbon and carbon dioxide. Once again, the carbon dioxide is recycled. The carbon chiselled off reaction tubes are the coal for small scale smelting of iron ore into iron. Not to mention, iron carbonyl refining is then possible.
https://www.youtube.com/watch?v=44OU4JxEK4k
Given enough iron and other metals, then iron catalyst for Fischer-Tropsch process on Mars is ready. Then make the hydrocarbons.
Given enough hydrocarbons and fluorine atoms on Mars, the Martian global warming process with PFCs Perfluorohydrcabons can begin.
All these reactions require thermal and electric energies; they can all come from nuclear reaction and later solar cells on Mars.
However, all the above does not mean committing human lives to an in-situ water and carbon dioxide resources NOW do not require any more work.
Offline
#17 2017-04-14 17:17:44
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
http://www.orbitetech.com/technology/th … fault.aspx
A Quebec company claims that its process can recover metals from oxides. As Martian "soil" is rich in magnesium, iron, titanium, silicon and aluminum, can its process be used?
Offline
#18 2017-04-25 02:07:49
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
https://upload.wikimedia.org/wikipedia/ … 121203.jpg
Hall–Héroult process is the major industrial process for smelting aluminum. Given that martian soil is very rich in metal oxides, can each metal be manufactured from electrolysis of its oxide, similar to the fate of aluminum compounds in the process? Further, in the Hall–Héroult process tetrafluoromethane/carbon tetrafluoride and hexafluoroethane are byproducts. Given enough Martian fluoride resources, can these exhaust gases be refined and dissipated to the Martian atmosphere close to the ground? Thus this dissipation contributes to perfluorocarbons global warming in the Martian terraforming.
Offline
#19 2017-06-16 08:20:38
- Antius
- Member
- From: Cumbria, UK
- Registered: 2007-05-22
- Posts: 1,003
Re: Ore resources on Mars
This is interesting: http://www.lpi.usra.edu/meetings/geomar … f/7031.pdf
We would need about 1000 tonnes of carbon to build a small Magnox gas cooled reactor (150MWth) on Mars. To make that much graphite using manufactured methane pyrolysis, would take about 17MW-years of electric power. A source of methane (or other organics) on Mars, would cut the required energy investment by at least 90%.
Last edited by Antius (2017-06-16 08:21:25)
Offline
#20 2017-06-16 11:14:01
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 7,936
- Website
Re: Ore resources on Mars
tetrafluoromethane/carbon tetrafluoride and hexafluoroethane are byproducts.
I gave a presentation in 2004 about smelting aluminum on Mars. My idea was a modification of the Bayer process to use ore confirmed to exist on Mars. It still required Hall–Héroult. After the presentation I discovered there's a company in Sweden already doing it; I re-invented the wheel! But good news is it works. My modification for Hall–Héroult is to recycle carbon anodes. Use RWGS (Reverse Water Gas Shift) to convert CO2 and H2 into CO and water, then simply continue to process to convert CO to carbon soot. Apply the carbon soot to the top of anodes, then apply pressure and heat to convert to low grade graphite.
Would that work with fluorocarbons? Or would the F-C bond be too strong?
Offline
#21 2017-06-18 07:56:42
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
Would that work with fluorocarbons? Or would the F-C bond be too strong?
Do you mean recycling the fluorocarbons? If so, where do the fluorine atoms go? I think your modification and fluorocarbons are two separte issues. Martian global warming needs fluorocarbons so byproduct exhaust is processed to release to the atmosphere.
Offline
#22 2017-06-18 08:31:08
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 7,936
- Website
Re: Ore resources on Mars
How much fluorocarbons are emitted vs CO2? Remember the Hall–Héroult starts with aluminum-oxide (Al2O3) and uses electrolysis to reduce that to aluminum metal. The oxygen combines with carbon of the anodes to form CO2. So there will be plenty of CO2. How much fluorocarbon per unit of CO2?
And what would happen if you took the gasses emitted from Hall–Héroult, run them into an RWCS reactor? No separation, just run everything into the chemical reactor? What would that do to the fluorocarbons?
Offline
#23 2017-06-18 17:03:10
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Ore resources on Mars
I do not understand what are asked.
https://en.wikipedia.org/wiki/Hall%E2%8 … ode_effect
However, the following is my imagination.
As hydrogen fluoride is corrosive, the exhaust is at first treated with premade carbon soot and in principle CF4 and hydrogen are yielded. CF4 is denser than CO2, CO and hydrogen. C3F8 is even denser. It is a liquid in Martian temperature when the other compounds are still gases. Therefore by controlling the how much premade carbon soot is used, C3F8 is produced. The idea is to create perfluorocarbons that collect many fluorine atoms. They are dense enough to be separated as liquids.
https://en.wikipedia.org/wiki/Octafluoropropane
Density 8.17 g/l, gas
Melting point −183 °C (−297.4 °F; 90.1 K)
Boiling point −36.7 °C (−34.1 °F; 236.5 K)
https://en.wikipedia.org/wiki/Hexafluoroethane
Density 5.734 kg.m−3 at 24 °C
Melting point −100.6 °C (−149.1 °F; 172.6 K)
Boiling point −78.2 °C (−108.8 °F; 195.0 K)
https://en.wikipedia.org/wiki/Tetrafluoromethane
Density 3.72 g/l, gas (15 °C)
Melting point −183.6 °C (−298.5 °F; 89.5 K)
Boiling point −127.8 °C (−198.0 °F; 145.3 K)
Fluoride is unlikely to be oxidized so using carbon soot -- as RobertDyck mentioned -- recovers hydrogen from hydrogen fluoride. Adding more hydrogen, running the Reverse Water Gas Shift Reaction, and producing water and carbon monoxide are possible as fluorocarbons are bystanders. At that stage of RWGS, the fluorocarbons are separated into another separate flow inside the factory. They are used as heat transfer refrigerants for the mildly endothermic RWGS. https://en.wikipedia.org/wiki/List_of_refrigerants
However, hydrogen fluoride is also recovered, converted to metal fluorides and reused in the electrolytic cells. So the competition between manufacture of perfluorocarbons from carbon soot and HF and that of metal fluoride from HF and metal oxides are mutually exclusive. Also, CO2 is still the majority of the exhaust. So perfluorocarbons are in much less amount even if they are made. Their use as heat transfer agents keeps them from uncontrolled escape into Martian atmosphere until enough amount is accumulated.
Last edited by knightdepaix (2017-06-18 17:07:55)
Offline
#24 2022-01-09 12:49:42
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Ore resources on Mars
Musk expects to be on Mars in 5 years or that SpaceX will take humans to Mars within 10 years, Elon Musk another timeline predicted
So what news do we see of the resources out there
Spacecraft discovers 'hidden water' in Mars Grand Canyon
https://www.cnet.com/news/spacecraft-di … nd-canyon/
Is thorium a new future of nuclear power? China Intends To Construct The World's First Thorium Fuelled Based Waterless Nuclear Power Plant
https://energycentral.com/news/china-in … ower-plant
Scientists Show That Algae Could Grow Using Only Mars Resources
https://futurism.com/the-byte/algae-grow-mars-resources
Could Thorium Become The Fuel Of The Future For Nuclear Energy?
https://wonderfulengineering.com/could- … ar-energy/
Offline
#25 2022-08-25 04:53:17
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Ore resources on Mars
New Mars Water Map Will Prove Invaluable for Future Exploration Missions
https://scitechdaily.com/new-mars-water … -missions/
There are Orbiters from Various Nations or International Space Agency groups checking the surface. Viking I, Viking II, Pathfinder, Opportunity Rover, and Spirit Rover checked what the 'soil' was made of and explored the surface, now a Chinese Rover Zhurong explores. Types of Irons, aluminium, magnesium, and titanium already found in the Martian soil, NASA JPL Rovers found nickel-iron meteorites sitting on the surface of Mars. Opportunity may have done Spirit, the affectionately named 'Oppy' found small structures, named "blueberries" which were found to be rich in hematite also a a vein of gypsum sticking out of the soil.
https://mars.nasa.gov/resources/6944
These are examples of the mineral concretions nicknamed "blueberries." Opportunity's investigation of the hematite-rich concretions during the rover's three-month prime mission in early 2004 provided evidence of a watery ancient environment.
Last edited by Mars_B4_Moon (2022-08-25 07:42:04)
Offline