New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2016-10-20 20:39:19
Aluminium Smelting in Space: What Would it Take?
Aluminium is the second most important industrial metal on Earth. Annual production in 2014 was 53 million tonnes. Our demand for Aluminium is nearly insatiable: Wikipedia suggests that Aluminium production has grown at a regular rate of about 5% per year since the 60s. In the last 25 years (The years for which I have data) the price has remained steady at around $1,775/tonne. For comparison, the bulk price of Pig Iron is around $200/tonne.
Aluminium is relatively expensive to produce because, unlike Iron, it needs to be electrolyzed instead of smelted. The energy costs of doing this are pretty large. Aluminium Oxide is a very chemically stable compound. At 100% efficiency, it would take 1670 kJ/mol of Al2O3, or 31 MJ/kg of Aluminium. At a more realistic 70% efficiency that's 44 MJ/kg. Energy costs for industrial use in the US average 7 cents per kWh. This works out to $856/tonne of Aluminium, which is most of the difference between Aluminum costs and Steel costs.
On Earth, this cost is typically reduced through the use of Carbon anodes. The reaction there is:
2 Al2O3 + 3 C + energy -> 4 Al + 3 CO2
This reaction requires about 22 MJ/kg at 100% efficiency instead of 31. At 70% efficiency and 7 cents per kWh, that's $604/tonne in energy costs. It's also worth noting that there are environmental costs to industry on Earth that are different or irrelevant in space.
Relative to Earth, Earth orbit is an energy-dense place. I've said this before, but by living on a rotating sphere you automatically reduce your insolation by a factor of pi. Atmospherics will reduce it even further. A high enough, slightly inclined Earth orbit will almost never be shaded and obviously won't have any atmosphere in the way.
This is why I have chosen Aluminium. I want to see what has to happen to use Aluminium production as a way to leverage the high energy density of Earth Orbit. Our total Aluminium production budget is $1000/tonne or $1/kg.
One important thing to recognize about our hypothetical Aluminium production business is that it has to be a bulk business. Aluminium is not valuable enough in small quantities to be worth producing. $1/kg is an aggressive target for a product coming from Space and I want to look at what it would take to make it happen.
-Josh
Offline
Like button can go here
#2 2016-10-21 00:22:21
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,371
- Website
Re: Aluminium Smelting in Space: What Would it Take?
What do you forsee as ore, and what's the application? Export to Earth? From Mars, Moon or an asteroid?
The reason I ask is the first step in ore processing on Earth is chemical. Bauxite is dissolved in strong alkali (sodium hydroxide), the liquid is then drawn off to another tank, then CO2 is blown through to create a weak acid (carbonic acid) to partially neutralize the pH. With pH close to neutral, aluminum hydroxide precipitates out. That precipitate is rinsed in clean water, then calcinated. That means baked at temperature higher than boiling for water, but less than required to melt the crystals. Hydroxide from the crystals combines with oxygen in air to form water. Since it's hotter than boiling, that leaves as steam. The final product is pure aluminum oxide.
At the 2004 Mars Society convention I proposed to reverse the pH to process feldspar. Bauxite is formed by a tropical rain forest. Water gradually converts feldspar and other minerals of basalt to clay, then trees and plants extract nutrients from soil including from clay. What's left over is bauxite. Mars never had a rain forest, so I propose using igneous rock. Anorthite and bytownite will dissolve in strong acid, including hydrochloric acid. Mars orbiters found lots of bytownite. Blow ammonia through to neutralize pH. The rest is the same. After the convention I discovered a mining company in Sweden is already doing it with a deposit of anothite. I have re-invented the wheel! Well, at least that proves it works.
Plagioclase feldspar is a continuum:
Albite = NaAlSi3O8
Oligoclase 70-90% albite, 10-30% anorthite
Andesine 50-70% albite, 30-50% anorthite
Labradorite 30-50% albite, 50-70% anorthite
Bytownite 10-30% albite, 70-90% anorthite
Anorthite = CaAl2Si2O8
The problem is when albite dissolves in acid, the silicon oxide does not dissolve. It remains behind as a layer of quartz. When a layer as thin as a soap bubble forms, it seals the grain so it can't dissolve any more. So albite is said to "etch" but not dissolve. Any plagioclase feldspar with too much albite does this.
So again, what's your source?
Offline
Like button can go here
#3 2016-10-21 19:44:09
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Aluminium Smelting in Space: What Would it Take?
Early production will be from the low hanging fruit of gathering and siple mining which will be refined but some processing but the finish product will be very heavy energy intense for Mars as we have no direct means to make the source of this energy.
http://primary.world-aluminium.org/proc … ation.html
Aluminium is highly reactive, which means that it forms very strong chemical bonds with oxygen; thus it takes a lot of energy to separate the two elements present in alumina (Al2O3). Typically, 13 to 15 megawatt hours (DC) is required to produce one metric tonne of molten aluminium.
Hall-Héroult process requires direct current (DC); therefore smelters transform AC into DC power using rectifier transformers, located within the smelting facility, for use in the aluminium reduction process.
150 pages to follow in this document which I have not the time to read but it may prove as a usefull reference. http://www1.eere.energy.gov/manufacturi … etical.pdf
the smelting units will not look like either of these next websites approach which are do it yourself cheap
http://www.instructables.com/id/Zero-Co … -No-Glue-/
http://www.instructables.com/id/Electric-Furnace/
I do see that Aluminum will an early processed metal for mars use but we will need to reasearch lower energy requirements to make it happen.....
Offline
Like button can go here
#4 2016-10-22 21:21:24
Re: Aluminium Smelting in Space: What Would it Take?
Re-reading my original post I suppose I should give a bit more background.
One of my biggest concerns for our future as a spacefaring civilization is that we as space enthusiasts haven't come up with a good, solid reason why the average person needs or benefits from Space.
Rather than proving it in some abstruse and ontologically questionable way, I'd rather say "We should go to space because it will make our lives better". In this case, a lower price of Aluminium will lower the cost of living and make someone a lot of money.
Therefore I'd like to try to ship refined Aluminium to Earth from Space.
Now, in terms of the origins of this material I'm not quite so sure. I was thinking that it should probably originate somewhere on the Moon and be processed in a high Earth orbit or perhaps a Lagrange point.
This is perhaps a time to urge humility. I will therefore quote GW Johnson so as to save him the time:
My contention is that we have not yet really "explored" the moon. No one has done deep drilling, no one has done much of anything [...]
Real "exploration" answers two deceptively-simple questions: (1) what all is there? and (2) where exactly is it? (There is an implied "are you sure about your results?" associated with them.) While that wording sounds like Texas slang, I mean them EXACTLY as they are worded.
Getting real answers to those two questions is a lot harder than most people think. This will require sending prospecting missions to sites all over the moon. These will need at the very least deep-drilling rigs.
You cannot answer the questions with remote-sensing (too unreliable, ground truth has been significantly-different nearly 100% of the time). Nor can you answer them by shuffling around in the surface dirt for a few pounds of loose surface rocks.
Having said that, we can be reasonably certain that there will be Aluminium ores somewhere on the Moon. But where?
Wikipedia suggests that the lunar highlands are mostly plagioclase feldspar. Obviously there will be substantial variation in purity but this means that there will be a mix of Sodium, Potassium, Calcium, Aluminium, and Silicon Oxides.
I'm not a geologist and I don't want to speculate any further. I will therefore make two broad claims and move on from there:
Somewhere on the Moon, more likely in the highlands but also very possibly in the Maria, there will be a substantial amount of economically viable Aluminium ore that can be extracted. If we're lucky, it'll be bauxite. If we're unlucky it'll be Kaolinite (Al2Si2O7). If we're absurdly lucky it'll be Cryolite (Na3AlF6).
We need further exploration to locate these resources and decide which are most economical to extract
From there, the ore will be processed and reduced to basic components ready to smelt on the Moon. This will be through either the Bayer process or through a different process.
I'm open to ideas regarding whether it makes more sense to process the ore on the Moon or in Space. On the Moon you would probably do it at a Peak of Eternal Light near the poles. In Space you would probably do it at EML4/5.
From there, the refined and pure aluminium would be shipped down to Earth.
-Josh
Offline
Like button can go here
#5 2016-10-23 05:13:15
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,994
- Website
Re: Aluminium Smelting in Space: What Would it Take?
I would think Luna would be best, given that smelting requires acceleration. Though you could use a centrifuge.
How do you intend to get the aluminium to the surface? You can't exactly make the aeroshell out of it, and importing them from Terra will kill your plan even if you're getting the ITS hypothetical launch costs. Shape it into a glider with a guidance system and send it down over a few days, avoiding the need for a heat shield?
I presume you're planning on using solar panels. How much will they mass for each tonne produced, given that they wear out fairly fast in space?
Use what is abundant and build to last
Offline
Like button can go here
#6 2016-10-23 08:58:21
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Aluminium Smelting in Space: What Would it Take?
my first thought for the moon would be to create and use a Fresnel lens furnace to smelt the ore....
http://www.dailymail.co.uk/sciencetech/ … -yard.html
focuses light into a beam
Beam can melt aluminium, meaning it is hotter than 660°C (1,220°F)
But the pair failed in their aim to melt bronze at 850°C (1,500°F)

http://www.instructables.com/id/Giant-F … t-in-Opti/
Offline
Like button can go here
#7 2016-10-23 13:00:20
Re: Aluminium Smelting in Space: What Would it Take?
I would think Luna would be best, given that smelting requires acceleration. Though you could use a centrifuge.
Good point here. We may want to look into the chloride process for the production of Aluminium. This process has been demonstrated but seems not to be favored on Earth. It seems that the technological hurdle blocking this method from being used is that the Aluminium Chloride needs to be completely anhydrous, and that it's very difficult to produce anhydrous aluminium chloride from oxide.
In general, if you have an oxide and want an anhydrous chloride, you would do as follows:
MO + 2 HCl -> MCl2⋅H2O
MCl2⋅H2O + Heat -> MCl2 + H2O
Where "M" denotes some arbitrary metallic element and the "⋅" symbol denotes that the water is chemically bound to the chloride in the form of a hydrate.
The problem is that with Aluminium, this tends to happen:
Al2O3 + 6 HCl ->2 AlCl3⋅1.5H2O
AlCl3 + 3 H2O + heat -> Al(OH)3 + 3 HCl
I propose that in the bone-dry vacuum of the moon the Aluminium Chloride Hydrate could be expected to slowly dry out as the water diffuses out of the sample (and is presumably recaptured for reuse nearby). It's easy to see that this wouldn't be cost-effective on Earth but on the Moon it might well be because instead of requiring a vacuum chamber the vacuum generates itself.
I should add that the chloride process uses ~30% less electrical energy than the hall heroult process.
By the way, Spacenut, take a look at the wiki article on Hall Heroult to see how Aluminium is produced now. It's technically an electrolysis process, not a smelting process.
How do you intend to get the aluminium to the surface? You can't exactly make the aeroshell out of it, and importing them from Terra will kill your plan even if you're getting the ITS hypothetical launch costs. Shape it into a glider with a guidance system and send it down over a few days, avoiding the need for a heat shield?
This one is the kicker, for sure. One thing we might want to explore would be a cast basalt aeroshell made from basalt in the lunar maria. It's obviously not acceptable to bombard the surface of the Earth with cannon-shells of aluminium, but we do need to figure something out. It might be worth looking into a momentum exchange tether orbiting in a medium Earth orbit to slow the Aluminium down a bit. This is probably only worth doing if it can be done using presently existing materials. I almost hate to say it, but perhaps some retrorocket thrusting is a good idea from there. Delivery is certainly the trickiest part of all this. Perhaps an inflatable heat shield? An entry trajectory like the one that was discussed for the X-33 (a series of shallow hops which slowly burn energy away before true entry) might be something worth investigating.
One nice thing is that we're somewhat less constrained when it comes to mass than we are normally, and we have no need to make the heat shield reusable. Furthermore, some level of product loss is acceptable so long as it does not result in harm to humans. GW, do you have any thoughts on this one?
A space elevator would be lovely, but I'd prefer not to rely on a megastructure that is about 50 years from being possible.
I presume you're planning on using solar panels. How much will they mass for each tonne produced, given that they wear out fairly fast in space?
I suppose the question is how much initial investment we should expect. I tend to prefer concentrated solar power using either steam turbines or stirling cycle engines, but it doesn't make sense to use these if you're not planning to make them on-site. One really nice thing about thermodynamic engines on the moon is that your cold-side temperatures can be really, really cold and so your efficiencies can be substantially higher than they would be in a corresponding system on Earth if your system is designed to take advantage of this.
It seems like we have developed systems with magnification factors of up to 1000x here on Earth, which means illumination of about 850,000 W/m^2. This is about 750x natural insolation levels on the Moon. Because there will never be a cloudy day on the Moon and because the Moon is barely inclined with respect to Earth's orbit we could probably even do better in space if our cooling systems can keep up.
Concentrating mirrors are made from Aluminium and Glass, both of which can be made from materials that are plentiful on the Moon. I would suggest that certain basic forming technologies using lunar resources are a prerequisite for this being a cost-effective way to produce Aluminium.
I would love nothing more than to provide you with numbers but I don't think I am able to at this time.
-Josh
Offline
Like button can go here
#8 2016-10-23 13:28:02
Re: Aluminium Smelting in Space: What Would it Take?
At a very basic level, Musk is claiming that his Interplanetary Transportation System will launch 300 tonnes in its reusable configuration at a cost of $62 million dollars. I'm highly skeptical of these numbers but he's claiming SpaceX can get launch costs down to $200/kg. This puts a hard ceiling on the ratio of downmass to upmass of 200:1.
-Josh
Offline
Like button can go here
#9 2016-10-23 17:34:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Aluminium Smelting in Space: What Would it Take?
Crushed bauxite is treated with moderately concentrated sodium hydroxide solution. Temperatures are typically from 140°C to 240°C; pressures can be up to about 35 atmospheres. High pressures are necessary to keep the water in the sodium hydroxide solution liquid at temperatures above 100°C. The higher the temperature, the higher the pressure needed. Aluminium oxide (sometimes known as alumina) is made by heating the aluminium hydroxide to a temperature of about 1100 - 1200°C.
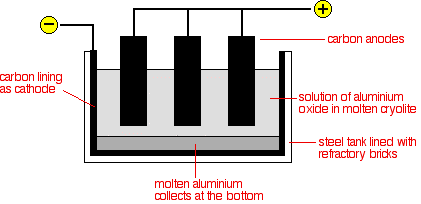
The cell operates at a low voltage of about 5 - 6 volts, but at huge currents of 100,000 amps or more. The heating effect of these large currents keeps the cell at a temperature of about 1000°C.
It looks like the Hall–Héroult process an electrolytic process. This aluminium smelter uses prodigious amounts of electricity; they tend to be located very close to large power stations. With first the aluminium oxide melted so that electricity can pass through it. The electrolysis is a purification process of the alumina, Al2O3, it is dissolved in molten synthetic cryolite, Na3AlF6, a Molten salt which is solid at standard temperature and pressure (STP) but enters the liquid phase due to elevated temperature to lower its melting point for easier electrolysis.
So by using the Fresnel lens furnace to melt the ore and to keep it molten it now make its less energy intense as we do not need the huge amount of amps to keep it liquid.....
Offline
Like button can go here
#10 2016-10-23 21:01:14
Re: Aluminium Smelting in Space: What Would it Take?
Electrical resistance in the cell tends to result in sufficient heating whether you want it to or not, and in any case thermal losses are small compared to the smelting energy
-Josh
Offline
Like button can go here
#11 2016-10-23 21:47:24
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,371
- Website
Re: Aluminium Smelting in Space: What Would it Take?
There are more steps to the first part of the Hall–Héroult process. I've described them before, do you want me to repeat? Basically the first part is a chemical process that separates aluminum oxide from other compounds/minerals in ore. Bauxite is a mixture of iron oxide, silicon oxide, aluminum oxide, as well as mud and debris because it's dug out of the ground. What's put in the electrolysis cell is pure aluminum oxide.
And electricity doesn't just heat the ore. Aluminum oxide is broken down via electrolysis. Cryolite is mixed with the aluminum oxide because pure aluminum oxide is not electrically conductive. Cryolite is sodum aluminum fluoride; it's electrically conductive and acts as a catalyst for this reaction. Electrodes are made out of carbon. That's not just because of the high current, oxygen from the aluminum oxide combines with carbon from the anode to make carbon dioxide. CO2 gas is released. That's why aluminum doesn't just re-oxidize.
Offline
Like button can go here
#12 2016-10-23 23:03:42
Re: Aluminium Smelting in Space: What Would it Take?
Hi RobertDyck,
I read your earlier post and appreciate your input in this topic, but as I said we don't have much specific knowledge about what our ores are actually going to be. If we don't know what we're trying to purify then we're getting ahead of ourselves if we start talking about how we're going to purify it.
-Josh
Offline
Like button can go here
#13 2016-10-24 08:25:32
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,371
- Website
Re: Aluminium Smelting in Space: What Would it Take?
On Earth we use Bauxite for aluminum ore. It's easy to harvest and plentiful. But bauxite is formed by a tropical rain forest. First water breaks down igneous rock to clay, then plants extract nutrients. After a tropical rain forest leaves no nutrients left, what's left over is bauxite. No rain forest, no bauxite. So Mars and the Moon don't have any.
They do have anorthite and bytownite, which are igneous minerals. Aluminum oxide can be dissolved from bauxite with sodium hydroxite, then precipitated with CO2. Aluminum oxide can be dissolved from anorthite or bytownite by hydrochloric acid, then precipitated with ammonia. Of course precipitation required gravity. So the question is what ore, and how are you going to extract it. It's a lot more involved than just heating.
Offline
Like button can go here
#14 2016-10-24 18:58:40
Re: Aluminium Smelting in Space: What Would it Take?
There are lots of different Aluminium minerals, from the very common to the very rare. There's a lot of Aluminium out there and there will certainly be viable ores somewhere. What the chemical and physical form of the Aluminium will be is impossible to know without exploring, so I think it would be better to put a box around this problem and figure it out once we've done some good exploration.
-Josh
Offline
Like button can go here
#15 2016-10-25 08:48:24
Offline
Like button can go here
#16 2016-10-25 15:42:11
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,136
- Website
Re: Aluminium Smelting in Space: What Would it Take?
The chemistry and technology are far outside what I know well enough to have an opinion on, but it sounds like some pretty common igneous rocks have attractive quantities of aluminum in them. Those would be anorthite and bytownite, as discussed above. Given the "right" chemical supplies and machinery (whatever they are), rocks go in one end of the process, and raw aluminum comes out the other.
Now, you need a bit of copper, zinc, or magnesium to alloy with your aluminum, and a place / equipment / machinery to create bar stock, sheet metal, forgings, wire, and tube stock out of those alloys, complete down to solution heat treat / temper / cold-work effects. There will be serious problems adapting what we know how to do, to vacuum / zero-gee conditions.
I see all this as neither trivial, nor impossible, to send into space somewhere. It ain't going to be cheap, but if there is a demand for aluminum products off-Earth, then it could be a moneymaker to manufacture those products out where they are needed.
Just an ignorant opinion of an old retired engineer talking way outside of what he knows well.
GW
Last edited by GW Johnson (2016-10-25 15:46:03)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#17 2016-10-25 21:48:10
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Aluminium Smelting in Space: What Would it Take?
That said we must go into space to do and try to do those things that we can do so well here on earth in the knowledge that it may just not work the same out there as it does here. Like you also said we will need to find "copper, zinc, or magnesium to alloy with your aluminum, and a place / equipment / machinery to create bar stock, sheet metal, forgings, wire, and tube stock out of those alloys, complete down to solution heat treat / temper / cold-work effects." solutions as well that will work in combination.... So pick a launcher solution, a carrier vehicle and see it land where we want it to go. Set the experiment in motion and see what comes out as finished product....
Offline
Like button can go here
#18 2016-10-27 07:30:07
Re: Aluminium Smelting in Space: What Would it Take?
My basic outlook on space exploration is that there's going to need to be a whole lot of money going up, so the people up there need to be able to send some money (or really goods or services that are worth money) back down.
We like to talk about space colonization like it's a given, but I would like to emphatically push back on that viewpoint. Space colonization is not a given. If we want it to happen and if we want it to be permanent we need to have a solid reason for it. Colonizing space will cost uncounted billions (trillions?) of dollars, and if all we have to offer in return is a warm, fuzzy feeling it's not going to happen.
Basically, I'm talking about trade. The colonies in space are going to be trading *something* (More likely, a variety of somethings) in the future in exchange for present and future investments in technology development, rocket launches, and equipment.
The cornerstone of trade is comparative advantage. Basically, in order to have a prosperous society in space, they have to have or be able to make something that people down on Earth want. I propose that we can leverage the higher energy flux (and to a lesser degree the hard vacuum) in space to produce Aluminium more efficiently and therefore have a comparative advantage over Terran production.
GW, I am of course interested in your take on any of this but I am most interested in your take on the delivery side of things. What's the cheapest way to do atmospheric entry when you're not limited by upmass, and when you stand to lose payloads but not people? Cast basalt tiles? Inflatable heat shield? Another way?
-Josh
Offline
Like button can go here
#19 2016-10-27 12:13:24
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,136
- Website
Re: Aluminium Smelting in Space: What Would it Take?
Hi Josh:
On trade: I think you have to go there and find out (1) what all is there, and (2) where exactly it is, before you can figure out what basis there might be for economic trade. Speculations are fine, but there's nothing trumps facts-in-hand (if you will forgive my choice of the word "trump"). That's what the exploration and adaptation/prospecting phases are for, that precede real colonization. My point: not knowing exactly how the economics will turn out is no excuse to not go and start looking. It's been the same throughout history.
On "easy" reentry: I'm assuming you mean one-shot deliveries of durable "somethings" to Earth from out in space. I don't know for sure, but the heat shield proposed and never done for the MOOSE (man out of space easiest) emergency bailout system was nothing but urethane foam, just thick enough to survive. It was kept in can, much like the foam sealer sprays in today's hardware stores. Blown into a form-fitting bag and allowed to expand, that was a combined personal heat shield and gee couch for a suited astronaut.
GW
Last edited by GW Johnson (2016-10-27 12:14:54)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#20 2016-10-27 15:23:12
Re: Aluminium Smelting in Space: What Would it Take?
Hey GW,
I'm one step ahead of you there ![]() . Your point is a good one, and it's well taken. We know virtually nothing about the availability of Aluminium ores on the Moon and we can't until we have some prospectors out there digging. We can easily speculate, but such speculation is meaningless without someone there on the ground.
. Your point is a good one, and it's well taken. We know virtually nothing about the availability of Aluminium ores on the Moon and we can't until we have some prospectors out there digging. We can easily speculate, but such speculation is meaningless without someone there on the ground.
Having said that, we can define a trade space of what's possible in terms of ore availability.
Our best case scenario is a large reserve of nearly pure, ore-grade alumina located at one of the poles near water, ammonia, methane, and frozen CO2.
An intermediate scenario is that there are medium-quality orebodies, perhaps Anorthite or Kaolinite, farther from the poles. There are any number of potential complications, of course.
The worst case scenario is that the Moon is an undifferentiated plain of various kinds of sub-ore quality rock and dirt.
The likely scenario is probably somewhere in between these but, as you're fond of saying, we have no idea where.
You're right, too, to say that ore availability is make-or-break for in-space aluminium production just like it is on Earth.
What I'm proposing is realistic speculation with clearly defined assumptions, as follows:
Following an extensive prospecting effort we discover that there are economically viable, minable deposits of Aluminium somewhere on the Moon
For reference, bauxite on Earth goes for about $50/tonne.
I like the looks of that MOOSE capsule. How stable are capsules on entry? Do they need guidance and retrofire, or will they remain stable without active control? How is the difficulty of entry related to the size of the capsule? I've heard parachutes are finicky. How precisely made do they need to be to work? Can you trust a parachute that hasn't been tested prior to use?
-Josh
Offline
Like button can go here
#21 2016-10-27 19:45:24
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,136
- Website
Re: Aluminium Smelting in Space: What Would it Take?
If the cg is further toward the heat shield than the center of pressure, the capsule is inherently stable enough to survive an unguided, uncontrolled entry. I've no criteria for how far ahead "ahead" is.
I don't remember about retrofire specifically, or the type of chute, but this was circa 1961, so it wasn't a supersonic ringsail. It would have been a small ribbon chute drogue at about Mach 1.5 to at most 2, and subsonic deployment of a big round chute for landing.
They never flew such a thing since there as no way to control where it came down within about a thousand miles, and in those days, no way to track where he actually landed.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#22 2016-10-27 20:30:07
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Aluminium Smelting in Space: What Would it Take?
To really have any commodity to be used as a returning for cash would mean that Earth has a shortage or the cost to the customer is so low as to undercut all others for its supply of it.
So far only He3 meets that criteria for experimentation here on earth and possibly rear earth magnetic materials. Everything else would cost more to go get than to which we could get from Earth its self.
Now trade from the moon to Mars thats another question all together different. This holds true for Venus, or the ateriod belt planetoids as these all hold what others need as they are short in them.
Offline
Like button can go here
#23 2016-10-28 07:19:43
Re: Aluminium Smelting in Space: What Would it Take?
If you want a farther-forward CG, a denser payload is definitely good. I definitely would hope for the parachutes to work every time but like I said some loss rate is acceptable, even if it's not desirable.
Before entry you need to get the Aluminium from the surface of the Moon to LEO, though. I propose that this should be done in three separate stages:
Moon to Low Moon Orbit.
Low Moon Orbit to Low Earth Orbit
Low Earth Orbit to Deorbit
Moon to Low Moon Orbit could be accomplished either with rockets or something fancier, like a railgun. Given the low lunar gravity, you might be able to get away with an NTR or some sort of lower thrust propulsion.
Low Moon Orbit to Low Earth Orbit will probably be done with a solar electric tug of some kind. This vehicle would be very reusable and would carry the cargo slowly from Lunar to Earth orbit. Upon reaching Earth orbit it will de-tatch and head back up towards the Moon.
Low Earth Orbit to Deorbit might not be its own craft. It might be part of the capsule, which fires a retro to re-enter. Or it might be a craft that slows down its cargo and then speeds back up again after dropping it.
If the cold traps on the Moon have useful amounts of volatiles the fuel will probably come from there. If not it'll have to come from elsewhere.
-Josh
Offline
Like button can go here
#24 2016-10-28 07:25:07
Re: Aluminium Smelting in Space: What Would it Take?
SpaceNut, trade doesn't only happen when there's a shortage. I encourage you to read the wikipedia article on comparative advantage. I believe that aluminium production in space could at some point become cost-competitive with production on Earth because of the cheaper energy.
-Josh
Offline
Like button can go here
#25 2016-10-29 13:07:26
Re: Aluminium Smelting in Space: What Would it Take?
Thinking about it more, I think the Moon Surface to Low Lunar Orbit segment is really key here. The delta-V (1.6 km/s) is not particularly high but rocket travel is going to be expensive.
One thing that might be worth looking at is a lunar space elevator. A lunar elevator would hang from the surface of the Moon going through L1, and would hang down to an altitude of about 85,000 km above the surface of the Earth. An object let go from this end of the tether would fall down to Earth (Actually it wouldn't even need to be at the end. You'd want it to be somewhere in the middle so that it just intersects the atmosphere, I think). I've got a couple of matlab/freemat scripts that can do these calculations if anyone is interested.
The tensile requirements on the cable are 26% less if you build a 500 km tall compressive tower as a base. Such a tower is no crazier than a 200,000 km tensile tether.
Even though it's possible with currently existing materials, a space elevator is a pretty long-term plan. Some kind of electromagnetic cannon is probably more doable and possibly comparably expensive. Rocket launch is the simplest technology with the smallest technical challenge and probably the highest cost.
Thoughts, anyone?
-Josh
Offline
Like button can go here