New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2015-12-05 03:49:52
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 4,000
- Website
Re: Potential Resources of Mars
Re. direct photolysis of water - this offers the possibility of piping in water to sunny areas and piping out methane. Even if both steps are 60% efficient, that's still far more efficient (36%) than photovoltaics (20%), and about the same if used in a generator at another 40% loss. Plus, once you have the hydrogen, making methane doesn't require much electricity - more heat, which can be provided by concentrated solar.
Use what is abundant and build to last
Offline
Like button can go here
#27 2015-12-05 12:48:56
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Terraformer said:
Re. direct photolysis of water - this offers the possibility of piping in water to sunny areas and piping out methane. Even if both steps are 60% efficient, that's still far more efficient (36%) than photovoltaics (20%), and about the same if used in a generator at another 40% loss. Plus, once you have the hydrogen, making methane doesn't require much electricity - more heat, which can be provided by concentrated solar.
I am guessing sooner or later someone here on Earth will implement such a process, in order to get some kind of "Carbon Credit", and to of course create a useful product. We will what to watch for such things.
Some things I notice about this site, is (1) when it comes to energy and chemical processes, they think in terms of solar panels and nuclear. This is understandable since it is high science, and also typically what the machines sent into space and to Mars use. (2) Typically they also want to recreate Earth on Mars, to the point they might bypass some factor Mars has to offer, simply because we don't do it on Earth.
To improve on #1, I suggest a greater awareness of what is going on in the world of alternative energy. I am not an especially perceptive person to now understand that the Hydrocarbon industry as we once new it is on it's way out. Curiously, the Gulf states might be a great place to look for advanced developments in solar energy. They are going to be living on a leaner budget, and are apparently aware that they are going to have to seek new methods. If they cannot produce their own innovation (I don't know if they can or can't), then they will hire it from the true East, and the West, so we will see many things develop there. They are of course involved with the Petrochemical industry, so I would be looking for them to integrate solar of all kinds with that existing industry. Another thing I have become aware of is that they are consuming more and more energy to produce water for their populations, and a very high cost. We should want to look at what they are doing and see if it has application to Mars.
For #2, I think RobertDyck is to most recreationalist, while some others are more open to exploring the unearthly characteristics that Mars has to offer. Both fields should be continued to be explored in my opinion. One field seeks to recreate the Earth on Mars, and the other seeks to exploit what Mars has to offer which is unfamiliar.
One thing about doing photolysis by certain methods, is that you could get both a chemical and a thermal benefit from the same machine, and that suits me well. If you want the truth, I want great benefit with small effort. That's potentially very profit oriented thinking.
Low hanging fruit that have been identified so far are (1) Day / Night and Seasonal temperatures. (2) Photolysis can be done with or without U.V., but I think U.V. offers a massive potential above visible light. (3) You might note that I have been talking about sand dunes. Apparently they can be made in part of Olivine, which under certain circumstances oxidizes in the presence of water and produces Hydrogen and possibly Clay. That is something I am interested in.
And their will be other things.
Of course I am often bothering you guys with lakes. It is because I see so much potential for them to serve the material needs of humans on Mars. And that can be by unconventional means I suggest.
For instance suppose RobertDyck's frozen sea exists, and it's location is inhabited.
What is serpentinization?
http://jersey.uoregon.edu/~mstrick/AskG … rry45.html
https://www.newscientist.com/article/dn … a-on-mars/

45 metres deep
The team of researchers, led by John Murray at the Open University, UK, estimates the submerged ice sea is about 800 by 900 kilometres in size and averages 45 metres deep. Images of the pack-ice-like plates can be seen in this PDF document, which was not embargoed when New Scientist first viewed it on 15 February.
So far, I think Imperial, so: 45m= 147ft 7.653546in
This should come is as perhaps ~1.350 bars of pressure on the average bottom of such a sea/lake.
So there is plenty of room to put a layer of undigested dune material on the bottom of the lake. The benefits I would hope to procure would be: (1) Oxidation of the material will produce provide for (a) Hydrogen (b) Clay. (That is what I hope for). I don't know what the rate of Oxidation at those temperatures will be, so it may or may not be a proficient method.
While my intentions for Clay are bricks, and radiation shielding, it turns out if you query for it clay is also useful for treating the body for contamination from radioactive chemicals. Just some more information. I am thinking that for certain situations, clothing with encapsulated wet clay (In plastic bubbles), might help to provide additional protection from radiation on Mars, as well, as even be useful to maintain body tone in the .38 g gravity field.
I also have of course other ideas about what to do with clay, for instance your Roman Arch buildings might be covered with wet clay with fibers incorporated. If the moisture could be maintained, this may provide a very good air tight seal. Sort of an "Air Dam" using wet clay counterweight, and also in concert with the added fibers, some tensile force to resist transient pressure deviations.
Back to the lake where you have put Olivine and other minerals from a dune on the bottom of the Lake and you presume H2 is bubbling out. You may now compress Martian atmosphere directly into the lake in proportion to Henry's law. There are some variables I will not go into detail here. So, now your lake has dissolved Martian atmospheric gasses in it, and H2 from the alteration of the dune materials. A biological system may operated living off of these chemicals. It should also occur that if you put CO2 into the water, it should become slightly more acid, and I speculate that will promote the conversion of dune material to H2 and Clay.
Should you really want to you might choose to "Frack" the floor of the Lake, and hope to actually create Serpentization driven Ocean Floor vents. But I would just try to use the dune material instead.
http://oceanexplorer.noaa.gov/explorati … obial.html
Methane-based life
People like to eat organic compounds. Pizza, peanut butter, and chocolate are among my favorites. Unfortunately, not much pizza or peanut butter is available to organisms living inside carbonate chimneys at the Lost City. Instead, significant amounts of methane and hydrogen gas are entrained within the fluids venting out of the chimneys. So, perhaps not surprisingly, within the chimneys there are colonies of micro-organisms that like to "eat" methane and hydrogen gas. Eating nothing but methane and hydrogen gas (especially without any organic compounds or oxygen) would kill you and me very quickly. Yet for these microbes, it's the best way to extract energy for growth and reproduction.
Lost City Biofilm
Colonies of microbes are attached to carbonate material from within the chimney walls. Some colonies form filamentous strands, such as the one shown in the far right of this microphotograph. Eash small dot (microbe) that makes up the strand is about 1 micron in size. Click image for larger view and image credit. (HR)We believe that a very high proportion of the organisms living within the chimney walls are using the hydrogen gas to make more methane. Organisms that generate methane are known as methanogens. The methane produced by the Lost City methanogens is consumed by other species of microbes. And just as organic matter synthesized by green plants from carbon dioxide, sunlight, and water provides food for you and me, the organic matter created by the methanogens feeds other species at the Lost City. Rather than using water and sunlight, methanogens create organic matter from carbon dioxide and hydrogen gas, and they do so at temperatures possibly reaching 90°C - 194°F!
Of course the lake bottom will not be hot like that, but I anticipate that their are bacteria which will do the job.
Anyway with just this you may have a source of organic chemistry. Plastics, and perhaps even food.
If your lake is briny, then you may have both an anoxic warm layer on the bottom, and a colder Oxidized layer on the top. This is due to an inversion possible from the relative lightness of a less briny cold layer over a more briny warmer layer.
So you might do this on the surface of your lake:
https://en.wikipedia.org/wiki/Solar_power_tower
So you can direct the heat from the tower into the lower layer of your lake, which will also provide a power generating ability as well, and;
you may at the same time: Do the direct photolysis of water and/or CO2 which you mentioned.
I suspect that at the ambient pressures of Mars you might get away with having the H2, Methane, and O2 mixed without serious explosion problems. If that proves true, then you can suck the gas out and mix it with the upper cold lake water, and that will provide a second method of chemical stimulation of the lake. The lake could have an ultimate result of the total consumption of the O2, if you just let the organisms convert all chemicals to body mass, and then let other organisms digest the resulting biomass against the O2, but if you interrupt the food web and extract the biomass, and convert it to Plastics, it is possible that you would be left with an O2 surplus in the lake, at least in the upper cold layer.
With all that, it might be nice to have a pleasant place to live. Some of that could be in the lake, some on top of the lake. Perhaps RobertDyck could loan us this:
Connection with the lake could be a vertical or diagonal tube from this dwelling to either the cold upper layer or the warm lower layer. It might provide an escape path if things go wrong topside, and will certainly provide access to the resources of the lake.
Last edited by Void (2015-12-05 14:10:07)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#28 2015-12-05 17:24:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
The dream of Earth on Mars Colonies, exploration and science are just that of course when the Funding for missions are not unlimited, the ability to launch the tonnage from Earth for the mission is limiting and for what we land on Mars is as well with its own limiting factors.
The quest for Mars will be by steps taken via robotic mission for site selection and of insitu resource availablity testing. With preloading of supplies and equipment at the selected site follow by a first mars mission. The future of man on Mars will be laid on that first missions success with the blend of science and colonization efforts to provide for follow on missions to the same site for further developement and question answering by science as to what we would want to learn from Mars.
That said we will need to be pretty good at recycling and building with next to nothing to make use of in those early missions with regards to creating equipment we do not have to making the materials and techiques to build our dreams on Mars. Sure mars will get easier with more missions and further preloading of materials, supplies and equipment to make life easier as well as the process to build a new Earth on Mars.
Online
Like button can go here
#29 2015-12-05 23:23:11
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,381
- Website
Re: Potential Resources of Mars
Ok. I had listed criteria for choosing a site on Mars, based strongly on criteria established by JPL. I had complained that there is no location on Mars that satisfies all criteria. Then others on this site pointed out the frozen pack ice does satisfy all criteria. They cited studies by the European Space Agency that claimed it isn't lava as NASA claimed, but is actually frozen pack ice. Ok. I accepted published papers by scientists with Ph.D.'s who worked for ESA. The result was this site on Elysium Planetia appeared ideal. Now I'm given credit for this site?
I have said many times that if claims by ESA prove true, then the site is ideal. However, those claims must be confirmed. If they are, then great! Let's go there. I would love for those claims to be true. That would mean the perfect site has been identified. Shores of the frozen sea are the ideal location to build a base. But others have argued for this location, I've just acquiesced. Now I'm given credit?
Last edited by RobertDyck (2015-12-06 07:25:39)
Offline
Like button can go here
#30 2015-12-06 06:10:31
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
I'm tempted to mess with you but I won't.
Good work.
I resisted because of the historical articles which indicated lava.
More recent work indicates that the manner of the freezing of the water or lava, indicates that it would not be lava.
I objected to water due to the shape of the craters in that picture. They are bowl shaped, and do not indicate a muddy ice "splosh".
It took me a while, but it occurred to me that those craters could have been there before the water was flowed into that location. They would have acted like dams, and very little or no water would have flowed into the craters. So, bowl shaped craters do not ultimately disqualify the possibility that there is a frozen sea there.
Finally recent articles I have read vastly upgrade the amount ice that may be buried under ground on Mars. This ice is fossil and was deposited billions of years ago.
With all that, sure I would say there are fair odds that the frozen sea is there. Such a sea suits my purposes as well as yours. It will be checked out before the settlement of Mars and confirmed or denied. If it happens that it is an unsuitable location after all, then for my purposes, I would anticipate an eventual secondary settlement at a higher latitude, which will utilize lake methods for habitation. Tom K. identified a giant slab of ice which can serve as a potential secondary target.
Tom K.'s alternate site:
http://newmars.com/forums/viewtopic.php?id=7288
My collected references about fossil ice in the rift valley and also the whole northern hemisphere of Mars.
http://newmars.com/forums/viewtopic.php?id=7291
I think that I could easily find that they will some day prove that Mars is as bone dry as they tried to put fourth for many decades, but from my current information, I anticipate that is vastly icy in it's Northern Hemisphere.
I prefer your site, but if it fails to satisfy, there are likely to be alternates.
Last edited by Void (2015-12-06 06:20:40)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#31 2015-12-06 08:15:51
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,381
- Website
Re: Potential Resources of Mars
The perfect bowl shapes are one reason why some scientists claim the frozen pack ice is in fact ice. They point out an impact in rock produces a rough crater, but those at Elysium Planetia are too perfectly round. The bowl shape indicates ice. And there is a "splosh" deposit around the rim of the craters. This indicates ice. And their determination that ice is 48 metres deep is based on craters; impact craters give us holes so we can see how deep the ice goes. Shapes on the surface look like pack ice, and scientists have stated lava doesn't form shapes like that. Pack ice does. The shapes clearly indicate there was pack ice at the time it formed. The question now is whether the ice has sublimated away leaving window blown dirt in the shape of the pack ice, or whether the ice is still there. If it's gone then the dirt is a fossil. I suspect if it had sublimated then the dirt would collapse, creating rough shapes. It shouldn't retain perfect pack ice shapes. And the bowl shaped craters are "splosh" craters, which have not collapsed either, they're perfect.
But, as I've said before, we need to send an unmanned probe to confirm the ice. I would like a rover like the one proposed by the president of the Canadian Space Agency in 2002. His idea was a rover the size of Spirit or Opportunity, not as big as Curiosity. And with a multi-segment core drill: 10 segments, each 1 metre long, so it can take samples 10 metres deep. His idea was to put sample handling equipment on the back of the rover, with multiple instruments to analyze the cores. He hoped NASA or ESA would partner, because obviously we need someone to launch it. Canada doesn't have a launch vehicle. And he hoped the partner would provide some instruments to analyze the cores. He even had an institute that specializes in mining technology design and build a prototype drill. I saw that prototype demonstrated at the 4th Canadian Space Exploration Workshop. Unfortunately Parliament didn't approve funding. Sounds good to me, let's send one to the frozen pack ice.
The crater that you say Tom found also indicates layers. The shape of the crater clearly shows 3 layers of material. The top appears loose, like soil. Then a layer that's firm, so it holds it's shape. Then a flat floor, with a small bowl shaped crater in the centre of that floor. The floor indicates a much more firm material, that only broke to form a crater where the energy of impact was sufficient to do so. Now the question is what are those 3 materials? One guess would be dirt on top, then permafrost, then bedrock. But that's just a guess.
And yes, I used the word "dirt". It doesn't have any organic matter, so soil scientists would call it regolith. But we've seen from several Mars probes that this loose regolith is wind blown particles that form dust as fine as loess, and clay. Good quality soil on Earth is formed by mixing loess, clay, and peat moss. You can compost chicken manure and plant matter like leaves and grass clippings to form top soil for gardening. Mars doesn't have moss, or chickens, or grass, so no organic matter. But considering it has 2 of the 3 ingredients of soil, just missing organic matter, can we call it "dirt"?
Last edited by RobertDyck (2015-12-06 15:42:39)
Offline
Like button can go here
#32 2015-12-07 17:15:25
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Well, thanks Robert. We do clash. Our backgrounds are skewed in different directions, and our means to objectives are different.
Often when I argue a point it may be on the basis of evidence procured from the internet, and that is not always correct or understandable.
I will take your word that those craters do indicate water. I was mislead by what I read.
There is some benefit at least from my end perhaps to come eventually by me trying to integrate your projected methods with what I have tried to propose. So, far, it has produced mutant ideas, that come in as impractical or impossible, but I have a feeling that eventually it will show profit.
My best projected offering I might propose would hybrid GW's mushroom houses with your structured thinking, and try to integrate them into a lake type real estate setting. As I have said it is not read for prime time. At the moment I am speculating on a situation where you have a "Mushroom House" with transparency on the sides. Instead of Regolith on the top however, a bag of water. Above that solar panels. The entire transparency should be surrounded by a robot which is also a reflective Heliostat which can spin around that circular window it envelopes, reflecting sunlight in from the leeward side of the sun source. The idea would be to make the mirror specifically reflective of visible light, and either absorbing of U.V. or fluorescent of it so that it converts U.V. to visible wavelengths. I also have a flower petal design in mind where the petals on hinges can cover the windows as the sunlight moves to directly intercept them. The petals would also be able to fold upwards to hold in heat at night. There again, the intent would be to greatly reduce the U.V. by selective reflectance of a mirror, and ideally incorporating a fluorescent effect to add visible light to what enters the greenhouse. In this way, I would hope to increase the lifetime of plastic films used as the transparent windows.
I think that in the age of robotics which is emerging it is not a bad pathway to investigate robot/heliostats with special reflective/fluorescent coatings for such a purpose, particularly if maintaining transparent windows with plastics is going to be such a economic load.
Anyway, back to the mushroom house structure, where I start with GW's design, and add a water tank on top, really the water tank is for counter pressure. As for the water within, perhaps you could do something biological with it, but really its real purpose is to provide counter-pressure, and to provide temperature moderations for the day/night cycles. The hot air in the greenhouse going to the ceiling during sunny days, and being absorbed, and at the night that heat being re-radiated into the greenhouse. Another value of using water, is it can be adjusted to some degree, as for the load on the ceiling, if that would have value.
As for the Mushroom, I actually add a long stem which is a passage down into the lake. Your mushroom could be pressurized to the degree you want, most likely lets say from 50-330 mb pressure. As in a barometer, the water column in the tube will respond with height appropriately. Your pressurization will depend on what you want to grow, and if shirt-sleeve humans/EVA Humans/Robots will work with the plants.
A polite response will be preferred, as I presented this as extremely proto-type and tentative, hoping that will be adjustable to practicality.
I have presented it for your entertainment as potential Fertilizer for the garden of your mind, sir.
------------------------------------------------------------------------------------------------------------------------------
In my other fantasy world, I am back to trying to exploit UNEARTHLY effects on Mars. As you may have read I am attempting to find a way of harnessing the powers of corrosion of dune materials to provide resources for settlers.
One other thing I am rather infatuated with is atmospheric separations. Apparently this has been looked into on this site and elsewhere. On this site I recall Antius having some involvement. Cryogenics were contemplated for that purpose. However they require lower temperatures than I would prefer to use.
So, I would like to see if there is a way to remove the CO2 from captured atmosphere, and deal with the remnant in some other way.
For the removal of the CO2, we might consider pressurization and the night time cold temperatures. Alternate Reverse Osmosis might be considered, (I think) squeezing all gasses out through a membrane, except CO2. Or the reverse, pumping atmosphere into a plastic bubble which allows the CO2 only to leak out. I believe we bumped into that potential in our conversation about ETFE which has been treated with embedded glass. (If I have that right).
Anyway, at least I want all the atmospheric gasses except the CO2 to go into a production stream. If the CO2 can be liquefied then it could easily be used to turn a turbine later to generate electricity, using heat from a lake, or solar energy.
I will get more direct. In particular I want to get the Oxygen out of the mix. At this time I am contemplating the use of Electrowinning.
https://en.wikipedia.org/wiki/Electrowinning
Please don't consider this rude, I just want to emphasize, that I am not contemplating Electrolysis.
There seems to be a persisting confusion on that, so I want to offer clarity for what I am after. I am not saying that I definitely understand how to get what I am after, but I do have a specific notion of what I am after.
It is obvious that corrosion will not produce free Oxygen, it will provide Hydrogen, and in the presence of a Carbon source (CO2 & CO), microbes might create Methane in the waters at the bottom of this lake. Of course the lake bottom itself will be food, but really not much surface area is presented that way. Adding dune grains to the bottom of the water will "Feed" the stomach of the lake. Lots of surface area. The food is well chewed ![]()
It may be possible to control the PH of the bottom water by the quantity of the dune material balanced against the amount of CO2 you dissolve in the water. Of course this would be done to aid digestion of the dune materials. My understanding is that the dune sands will rapidly alter in the presence of liquid water. (Rapid as a unit of time is poorly defined in that description). But if the result were to resemble the serpentinization of Olivine and the metals in the dune materials which may not be completely Oxidized yet, I would expect to produce salts of metals dissolved in the slightly acid water. Additionally as stated before H2 should result and I also hope that CLAY will result.
https://en.wikipedia.org/wiki/Olivine
Well, here is some interesting material, not quite mentioning clay, but giving some time definition for one pathway for the decay of Olivine.
A worldwide search is on for cheap processes to sequester CO2 by mineral reactions, called enhanced weathering. Removal by reactions with olivine is an attractive option, because it is widely available and reacts easily with the (acid) CO2 from the atmosphere. When olivine is crushed, it weathers completely within a few years, depending on the grain size. All the CO2 that is produced by burning 1 liter of oil can be sequestered by less than 1 liter of olivine. The reaction is exothermic but slow. To recover the heat produced by the reaction to produce electricity, a large volume of olivine must be thermally well-isolated. The end-products of the reaction are silicon dioxide, magnesium carbonate and small amounts of iron oxide.[22][23]
Oh, and I do believe that the reaction also creates heat, so it will help heat the lake.
Back to Electrowinning. I believe that metals can be extracted to the cathode of the device from the lake waters. It may be that dissolved Hydrogen and perhaps Methane can be concentrated that way as well.
In this case I am not aiming for the Oxygen we might want yet. At this time I am contemplating the use of Electrowinning.
https://en.wikipedia.org/wiki/Electrowinning
Please don't consider this rude, I just want to emphasize, that I am not contemplating Electrolysis.
So, we have our Cathode(s) in the bottom of the lake, and we are attempting to collect dissolved Metals/Hydrogen/Methane on the Cathode(s).
Perhaps we could try to put the Anode(s) in the upper waters, and hope to extract dissolved Oxygen. But there will not likely be any unless we put it there.
If we have treated Martian atmospheric gasses by removing the CO2 (Or as much as suits us, since it is going to get used in the corrosion process down below), we can add other gasses, including Oxygen.
I believe that their is about twice as much Oxygen as Carbon Monoxide in the mix, and that N2 and Argon will be added as well. So, I would hope to collect some of the Oxygen on the Cathode(s). As for the Nitrogen and Argon and Carbon Monoxide.
The Carbon Monoxide might be digested by Micro-organisms in the lake. They might use Oxygen, or H20 as the Oxidizer.
As for Nitrogen, I believe that in Hypersaline cold lakes an interaction occurs where Nitrous Oxide is produced. I don't know what would be done with that or about that, but perhaps somewhere in the process you could extract both purified N2, and Nitrous Oxide. As for the Argon, isn't that inert?
Maybe really you would subject water which has the Oxygen and Carbon Monoxide exhausted by Electrowinning or microbes, subject that to a vacuum and by Henry's laws extract a N2 and Argon Mix.
So, a cyborg ecosystem that runs on Corrosion and breaths Martian air. (And is quite adaptable to hybridize with solar power options).
Last edited by Void (2015-12-07 18:28:41)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#33 2015-12-08 14:54:00
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Well, for a while since the last post, I have been thinking about this.
I think it might be possible to extract organic materials from dune materials at the bottom of such a lake, or dune materials in a process line of our making in the lake.
The most optimistic outcome being the extraction of a food resource from such a "Mud".
The least optimistic being the extraction of fuel resources.
Between those, the possibility of raising mushrooms on dune soil that has been enriched by organics.
What about tilling the soil at the bottom of the lake, aerating the soil, and also extracting H2, Hydrogen, Nitrous Oxide, and perhaps even organic matter.
Adding CO2, N2, and CO for instance, during the tilling.
Another alternative would be to wait for such soil to accumulate organic matter, and then take it and raise mushrooms on it.
Point being you might really get a lot of needs satisfied. Maybe you would not need as many crops raised under photosynthesis. (Which might make living on Mars easier profit wise).
And if you might contemplate the above, perhaps you can think of a process line with tanks and pipelines, etc. You get it factory level stuff.
I though I might have a lot more to say, but my me is going south of the border. Maybe later.
Last edited by Void (2015-12-08 15:06:28)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#34 2015-12-08 20:19:51
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
If we save all the food scraps, paper products and human waste from the trip out and drop them to the surface in a lander we would have all the organic materials that we would need to start the process of plant growth.
Online
Like button can go here
#35 2015-12-08 21:47:55
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Please join the conversation Robert.
Spacenut, OK, I will sidestep from the Mega Futurist Large Lake city and try to be helpful to answer Roberts question of soil with you. It is important to provide for vigor for the first small community, so that the following much larger presence can be parented well by it.
Here I offer this. Yes your organic materials, really as ugly as it may be even all human waste. I am squeamish however for certain things, I would really want to see some kind of hygienic sterilization process, and then to see what we can do with mushrooms.
http://www.gmushrooms.com/MushroomInfor … osting.htm
http://nrcmushroom.org/Folder_Recycling_SMS_Folder.pdf
And really, why not? No lights needed except to set up, inspect, and harvest. And a contribution to food sources cannot be dismissed as unwelcome.
Later, I will return to what I was talking about, and we will see if anyone is even willing to acknowledge that I am posting the materials, but for now starter colony soil.
Last edited by Void (2015-12-08 21:53:34)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#36 2015-12-09 01:19:51
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,381
- Website
Re: Potential Resources of Mars
Ok, we want to extract gas from Mars atmosphere. I gave a few presentations at the Mars Society convention of 2005. One was "ISRU atmosphere harvesting". My idea was to collect everything but CO2. Robert Zubrin and his employees came up with the Mars Atmosphere Carbon Dioxide Freezer (MACDOF), which used the cold of Mars to create dry ice. Mars is so cold at night that the device only requires a few degrees more to freeze CO2 to form dry ice, probably as frost. His idea was to seal the container at dawn, then warm it to sublimate the dry ice. Because it's sealed, the phase change will cause CO2 to self pressurize. It takes minimum energy because it uses the fact Mars at night is so close to the freezing temperature of dry ice. Ok, that's good, but I want to harvest everything else.
My idea starts with a pump to pressurize a canister with Mars atmosphere. This requires a pump, and that pump will require a lot of power. This won't be as energy efficient as the MACDOF. Mars ambient as measured by Mars Pathfinder varied from 6.77 to 7.08 mbar (Pa). Curiosity rover is a different location, lower altitude; it measured 7.5 to 9.2 Pa. My device will require pressure of 10 bar, which is more than 1,000 times Mars ambient. A freezer coil in the bottom of the canister will chill the air to -100°C, accumulating dry ice. As CO2 is removed, it will drop the pressure in the canister. A pressure sensor will activate the pump, adding more Mars atmosphere to maintain 10 bar. The idea is to keep everything else. This gas mix can be added to oxygen to create air for the habitat. Because it is used to dilute oxygen, it's called diluent gas. This diluent gas will be mostly nitrogen and argon, but will have other gasses from Mars atmosphere. Dust will be filtered out at the intake to the pump.
The problem is Mars atmosphere has ozone and carbon monoxide. If nothing is done, after removing CO2 the CO will be so concentrated it will be lethal. So a rhodium based catalyst is added to the top of the pressure canister. It's the same canister used to freeze out CO2, because the catalyst combines CO with O2 to form CO2. Since this is the same canister, the CO2 produced by the catalyst will be removed as well. Mars doesn't have much O2, but it doesn't have much CO either. In fact it has more than enough O2 to decompose all CO. On top of that, the same rhodium catalyst will decompose ozone: 2 O3 → 3 O2. So this makes more O2 even as O2 is combined with CO. Mars doesn't have much ozone, but all together there will be roughly 4 times as much O2 as required to decompose all CO.
Another catch with this system is that the catalyst requires +24°C to work. So the catalyst must be warmed even as the freezer coils at the bottom of the same canister chill to -100°C. This will consume even power.
Once the pump stops activating, pressure is stable at 10 bar, and the CO sensor registers absolutely no CO within detection threshold, then the intake valve will be sealed, pump turned off, and heater to the catalyst turned off. The freezer will stay on. As the catalyst cools, the chamber will chill further, which will further reduce pressure. Once the pressure stabilizes, then gas in the chamber will be released into a much larger holding tank. This release of pressure will cause dry ice to sublimate, so the release will be quick. Once pressure is relatively stable, the valve between tanks will be sealed again. And the pressure canister will start another batch.
Remaining pressure can be vented to Mars, then the canister sealed. Then the freezer coils can reverse to heat the dry ice, sublimating it. We have a block of dry ice frost, may as well keep it. Phase change will pressurize CO2 gas, which can be added to the CO2 for ISPP or ISRU. Then vent the canister again, seal, and you're ready for another batch.
Another pump will pressurize gas from the release chamber to a holding tank. The release tank must be pumped down to laboratory vacuum in preparation to receive the next batch.
Diluent gas produced this way will be: 61.0% N2, 36.1% Ar, 2.1% O2, 0.74935% CO2
Trace gasses: 560 ppm Ne, 68 ppm Kr, 18 ppm Xe
The device is not intended to remove water, but it will. Water will freeze out so thoroughly that vapour pressure has to be expressed in scientific notation.
Note: If oxygen is added to this and used for habitat air, the CO2 will be a bit too high. Even after diluting with O2, it will still be too high. But a freezer is best used to remove the bulk of CO2, it can't effectively remove the remaining trace. To remove that, you need a sorbent. The regular life support system of a habitat uses a regenerable sorbent, so just use that. This means freshly produced air will smell stuffy until the life support system has been run for a while. This level of CO2 is not toxic, it'll just smell stuffy.
Last edited by RobertDyck (2015-12-09 03:38:50)
Offline
Like button can go here
#37 2015-12-09 01:27:58
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
I like it. Good stuff.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#38 2015-12-09 02:11:28
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,381
- Website
Re: Potential Resources of Mars
This diluent gas can be processed further. You can use it as is for habitat air, but can do further processing. There are basically two options: membrane to produce N2, or burn with hydrogen to produce ammonia.
Here on Earth there is a company selling semipermeable membranes to separate nitrogen from air. This equipment is sold to automobile service centres (garages) to fill tires. They claim it extends the life of your tires. Not sure that's true, but the equipment exists. This same membrane system can be used to separate nitrogen from diluent gas. That is without adding oxygen. The membrane is hollow tubes, finer than a human hair. It requires pressure, so energy to run the pump. Reducing entropy requires input of energy.
Another option is to add hydrogen and burn in the presence of a catalyst. Oxygen will burn to form water, hydrogen will combine with nitrogen to form ammonia. But first, you must remove any trace of CO2. That must be done with a sorbent. If any CO2 is present, H2 will combine with CO2 to form water and CO. Once you have ammonia and water, they will combine to form hydrous ammonia: NH3 + H2O → NH4OH. The Haber process uses an iron based catalyst under high pressure and temperature. You can separate this out by freezing at -77.7°C, it will freeze ammonia as a solid. Remaining gasses will be noble gasses: 99.9% Ar, and traces of Ne, Kr, Xe. There will also be traces of ammonia and non-combusted nitrogen. If you get the balance right, and freeze out all ammonia, it will be 99.98% Ar, 0.0156% Ne, 0.00187% Kr, 0.00050% Xe. This commercially pure argon can be used to fill the gaps between panes of sealed windows.
If the goal is to produce nitrogen fertilizer for a greenhouse, ideal is ammonium nitrate. That is formed by combining anhydrous ammonia with nitric acid. So the ammonia has to be heated to form a gas, and a desiccant used to remove moisture. The reaction is violent, highly exothermic. Excess water is then evaporated, the resulting ammonium nitrate melted and formed into pellets. Commercially, the pellets require a coating to keep them from sticking to each other.
Nitric acid is formed by combining nitrogen dioxide with water. This can be done by simply bubbling the gas through water.
3 NO2 + H2O → 2 HNO3 + NO
Nitrous oxide produced will combine with oxygen in air to produce more nitrogen dioxide.
Be careful with nitric acid. It's highly corrosive.
NO2 is formed by oxidation of NO. That is formed by: N2 + O2 → 2 NO
This happens by an electric arc at >2,000°C
Ok, there's an easier way to produce nitric acid. But I don't think I should post it.
Last edited by RobertDyck (2015-12-09 02:33:47)
Offline
Like button can go here
#39 2015-12-09 10:53:35
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Fine information. So, what the soil needs, it can get.
And we can consider air to be in part available by the means you have described.
I suggest that for that to deal with CO, you might present the mix to a volume of water where an organism that can digest CO can be grown. It would consume some of the Oxygen, but get rid of the CO, and the biomass might have value.
Last edited by Void (2015-12-09 10:55:56)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#40 2015-12-09 21:37:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
RobertDyck has laid out a nice sequence of uses with each feeding the next with minimal energy being put into the processes to get the needed output from the source of its inputs.
There are other uses as well for the Co such as in a Carbon monoxide/oxygen engine as we have discussed here as well. https://en.wikipedia.org/wiki/Carbon_mo … gen_engine
We can also use it in Water-gas shift reaction https://en.wikipedia.org/wiki/Water_gas_shift_reaction to form carbon dioxide and hydrogen.
http://www.chem.tamu.edu/rgroup/goodman … 71262z.pdf
CO + O2 and CO + NO Reactions over Pd/Al2O3 Catalysts
Here are some more useful reactions
http://www.diyspaceexploration.com/prep … -peroxide/
Online
Like button can go here
#41 2015-12-10 01:07:03
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
Very good to have.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#42 2015-12-12 20:27:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
Online
Like button can go here
#43 2015-12-13 12:32:17
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
We are Duned!
http://www.engadget.com/2015/12/11/curi … and-dunes/
http://www.bing.com/images/search?q=mar … ORM=IQFRBA
http://www.jpl.nasa.gov/spaceimages/det … d=PIA20171
This view of the undisturbed surface of a Martian sand dune called "High Dune" visited by NASA's Curiosity rover shows coarse grains remaining on the surface after wind removal of smaller particles.
So, what I am seeing in the above suggests that we could entertain various separation methods, in hopes of gaining concentrations of materials we might want. Obviously a vertical column of air flow could do a separation of sorts, although I don't know what that gains you.
Dry or wet magnetic separations might also work. Of course there could be wet flotation methods, and I am wondering if you could do a centrifuging of the materials. If you blew air into the centrifuge to levitate and fluidize the materials, could you get it to settle out in layers, where the layers might have a concentration of particular types of grains?
Of course size and specific gravity will matter in such a separator. So, you will likely like some kind of screens as well, since heavy materials of small grain size might float into the location of lighter materials with a larger grain size (Response of air friction to grain surface area, and of course the specific gravity of grain materials.
But it seems hopeful to me.
http://www.dailymail.co.uk/sciencetech/ … Earth.html



Of course the lighting tricks that are used leave you wondering if you are seeing reality, but I think I see enough color variations to suggest that you might even selectively extract patches of materials to get closer to what you want.
Last edited by Void (2015-12-13 12:52:55)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#44 2015-12-13 13:02:35
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
So, the dune materials themselves, if I can find anything.
Martian "Soil", which isn't quite dunes, but might be close:
https://en.wikipedia.org/wiki/Martian_soil
The difference in the concentration of dust in Earth's atmosphere and that of Mars stems from a key factor. On Earth, dust that leaves atmospheric suspension usually gets aggregated into larger particles through the action of soil moisture or gets suspended in oceanic waters. It helps that most of earth's surface is covered by liquid water. Neither process occurs on Mars, leaving deposited dust available for suspension back into the Martian atmosphere.[26] In fact, the composition of Martian atmospheric dust – very similar to surface dust – as observed by the Mars Global Surveyor Thermal Emission Spectrometer, may be volumetrically dominated by composites of plagioclase feldspar and zeolite[27] which can be mechanically derived from Martian basaltic rocks without chemical alteration. Observations of the Mars Exploration Rovers’ magnetic dust traps suggest that about 45% of the elemental iron in atmospheric dust is maximally (3+) oxidized and that nearly half exists in titanomagnetite,[28] both consistent with mechanical derivation of dust with aqueous alteration limited to just thin films of water.[29] Collectively, these observations support the absence of water-driven dust aggregation processes on Mars. Furthermore, wind activity dominates the surface of Mars at present, and the abundant dune fields of Mars can easily yield particles into atmospheric suspension through effects such as larger grains disaggregating fine particles through collisions.[30]
The Martian atmospheric dust particles are generally 3 µm in diameter.[31] It is important to note that while the atmosphere of Mars is thinner, Mars also has a lower gravitational acceleration, so the size of particles that will remain in suspension cannot be estimated with atmospheric thickness alone. Electrostatic and van der Waals forces acting among fine particles introduce additional complexities to calculations. Rigorous modeling of all relevant variables suggests that 3 µm diameter particles can remain in suspension indefinitely at most wind speeds, while particles as large as 20 µm diameter can enter suspension from rest at surface wind turbulence as low as 2 ms−1 or remain in suspension at 0.8 ms−1.[25]
So, then perhaps electrostatic and van der Waals forces can be used to separate the materials to some extent as well.
Chameleon foot stickiness, vs magnetism, electrostatics, centrifugal force, etc.
The Soviets left this behind for us it seems:
http://encyclopedia2.thefreedictionary. … omagnetite
Titanomagnetite deposits (basically magmatic) occur in association with ultrabasic, basic, and alkalic rocks; they also occur in placers. Titanomagnetite is used in producing iron, titanium, and vanadium.
And here is a method (Perhaps not the method) to extract materials:
http://www.sciencedirect.com/science/ar … 6X14001716
Abstract
A novel process for the extraction of iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates is proposed. This process involves several steps: partial reduction of the concentrates, magnetic separation, hydrochloric acid leaching of the titanium-bearing tailing, and alkaline desilication of the HCl leach residue. The partial reduction ensures that the vanadium and chromium are predominantly concentrated in the titanium-bearing tailing. Subsequently, magnetic separation is used to recover an iron concentrate with a total iron content of 94.57%. During acid treatment, 90.8% vanadium leaching and 93.4% chromium leaching were obtained, with titanium losses of less than 0.3%. 96.3% of the silicon was removed by alkaline desilication, and titanium-rich slag with a purity of 93.39% was produced. The total recoveries of iron, titanium, vanadium, and chromium under the experimental conditions were 88.3%, 93.7%, 81.7%, and 84.4%, respectively.
I highlighted a fragment about silicon, which I would have to think would be wanted as a material as well.
Last edited by Void (2015-12-13 13:23:31)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#45 2015-12-13 13:28:43
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
So, yes your articles about various deposits are important, but I think that in the beginning it will be fortunate to have 2 or 3 significant materials available in a first settlement at a reasonable distance.
Being able to get stuff from dunes can help you increase the spectrum of available stuff.
Further, I believe from previous reading that micrometeorites survive to enter the "Soil" of Mars. To me that suggests that some Nickel should be present, although it is not mentioned so far.
Also of a great interest to me is to create a hot solar process to create Hydrogen and perhaps even Clay from some part of dune materials.
I have found from reading that basalt materials could oxidize in the presence of water over a number of years, and might change to clay.
I would like to see if you could cook the stuff at a high temperature and get out Hydrogen and Clay in a batch. This could be very useful for the first settlements.
But I am somewhat confused and unsure:
https://en.wikipedia.org/wiki/Serpentin … _reactions
Serpentinite is formed from olivine via several reactions, some of which are complementary. Olivine is a solid solution between the magnesium-endmember forsterite and the iron-endmember fayalite. Serpentinite reactions 1a and 1b, below, exchange silica between forsterite and fayalite to form serpentine group minerals and magnetite. These are highly exothermic reactions.
So the reaction creates heat, and Hydrogen I believe, and perhaps even clay in some cases?
What happens if you add heat, as in solar heat? Do you get a quicker chemical reaction and perhaps even more Hydrogen?
Once again:
http://space.io9.com/sand-dunes-are-rai … 1678443555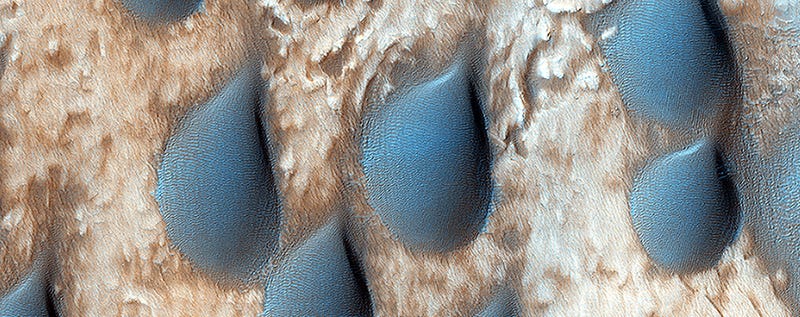
It's raining dunes! Well, no, not really, but these olivine-rich sand dunes on Mars really do look like classic cartoon drawings of raindrops sliding across the landscape.
Pyroxene and Fieldspar may also be present, I am not sure what reactions they will have.
So, I would have hopes that Hydrogen could be obtained in batches at will and perhaps "Burned" in fuel cells against a concentrate of Oxygen and Carbon Monoxide extracted from the atmosphere.
Otherwise, if that can't work of course you would want Hydrogen for various other reasons as well.
Last edited by Void (2015-12-13 13:54:13)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#46 2015-12-13 17:30:51
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
I see that the easy picking are those that require the least amount of energy to gather from the surface.
Filling in the chemical make up....
Pyroxene minerals
https://en.wikipedia.org/wiki/Clinopyroxene

https://en.wikipedia.org/wiki/Feldspar

Weathering of Common Rocks
Types of Chemical Weathering Reactions
http://www.tulane.edu/~sanelson/eens211 … nerals.htm

https://en.wikipedia.org/wiki/Olivine
https://en.wikipedia.org/wiki/Mineral_processing
By going for the dunes we avoid the crushing step in processing to a sand like grain.
Wet seperation of gold on earth in a pan required lots of water.
Industrial processing
http://www.pieralisi.com/media/files/14 … rators.pdf
heavy minerals separation physical properties
http://www.ironoremining.org/solutions/ … roperties/
SEPARATION OF SWELLING CLAY MINERALS BY A CENTRIFUGAL METHOD
http://www.clays.org/journal/archive/vo … -1-407.pdf
Electrical Separation of Mineral Raw Materials
http://eprints.nmlindia.org/4649/1/6-_D_D_Mishra.PDF
Online
Like button can go here
#47 2015-12-13 21:08:40
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,297
Re: Potential Resources of Mars
A wonderful body of knowledge Spacenut.
It reassures me that in fact there is far more that can be done than what I speculated. Dune material is a mishmash of stuff it would seem, but it may have quite a few of the things that will be wanted. And with what you have listed they can actually be obtained.
Wonderful!
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#48 2015-12-14 16:05:19
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Re: Potential Resources of Mars
What about Uranium? I would suspect that with one third gravity, Uranium would be easier to mine because we can dig deeper than on Earth. Uranium would be a local resource, we'd have to find out where to mine it, and then to concentrate the right isotope that can be used in reactors. With Mars you have weak sunlight, a thin atmosphere so not much wind power to extract, nothing burns and their are no fossil fuels, but one can still split atoms. Maybe we could power pollution factories who's purpose is to add greenhouse gases to the atmosphere. We also need to liberate gases from the soil so as to thicken the atmosphere, all that will require energy, so why not use nuclear to do it?
Offline
Like button can go here
#49 2015-12-14 17:17:06
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,381
- Website
Re: Potential Resources of Mars
What about Uranium?
The orbiter called "2001 Mars Odyssey" mapped several Mars resources. One was thorium. The reason is thorium is an indicator mineral for uranium; meaning go here to do further prospecting.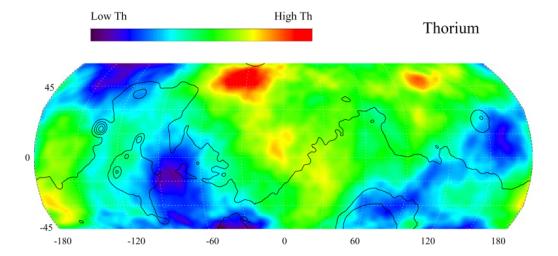
However, I believe we can use thorium itself. On Earth, there's 3 times as much thorium as uranium, and 100% of thorium in nature can be used as reactor fuel, while only 0.72% of uranium is U-235. The issue was Canada has Earth's largest deposits of uranium, and the United States has the second. There's more uranium here than thorium. And you can't make a bomb from thorium, while you can from uranium. The fact a thorium reactor is much safer, and it's almost impossible to use it for weapons manufacturer, is one reason it should be developed. But US military is not interested for exactly those reasons. India developed a thorium reactor to power a small town. One reason is they have much more thorium than uranium; I think they have the largest thorium deposit on Earth. So why not just use thorium itself?
...concentrate the right isotope that can be used in reactors.
100% of thorium in nature is ²³²Th. That's the correct way to write it. The only superscripts built into the standard font are '2' and '3', so I don't have to write it as Thorium-232.
Offline
Like button can go here
#50 2015-12-14 19:34:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,479
Re: Potential Resources of Mars
We have a topic Thorium reactors are go! (In India)
We have talked about the use to lessen the mas the Mars as well for the future missions such as in this topicGoing to Mars To stay - How Much Mass To LEO
Online
Like button can go here