New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2014-04-14 05:32:18
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
LOX/CO2-Diborane rocket for reusable landing boats
I found this interesting article of Zubrin about CO2-Diborane rocket for MAV, that has an exaust velocity of almost 3 km/s with an O/F ratio of 2.5.
Zubrin's idea is to bring diborane from Earth, get atmospheric CO2 from Mars, and use it as oxidizer for orbital ascending.
http://www.google.it/url?sa=t&rct=j&q=& … 2518,d.Yms
On wikipedia, I also fond that diborane, with LOX as oxidizer and an O/F ratio of 1.96 has an exaust velocity of almost 4 km/s, second only to LOX-LH2 rockets.
http://en.wikipedia.org/wiki/Liquid_rocket_propellants
So we can imagine a resuable landing boat like GW's design, with single fuel-dual oxidizer rockets, that use LOX-diborane for the descend, get CO2 from Mars atmosphere, and use it for ascend, saving a lot of mass, but without complication and energy needs of chemical processing: only a compressor to get CO2 and to store it as a liquid.
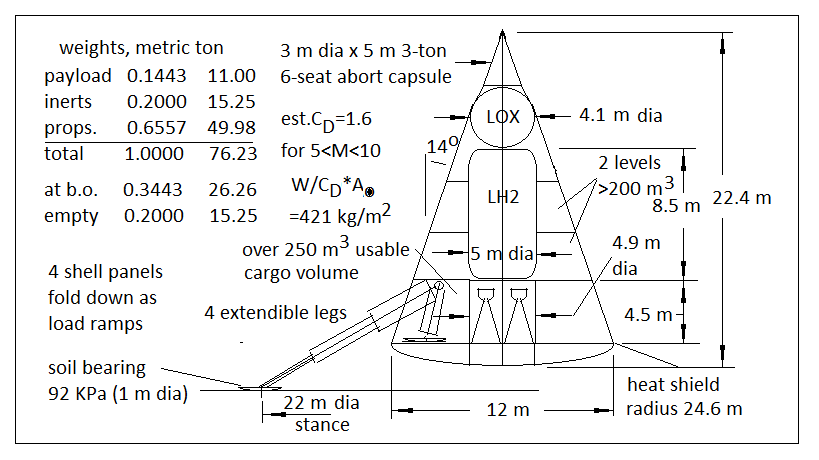
Last edited by Quaoar (2014-04-14 06:15:38)
Offline
Like button can go here
#2 2014-04-14 09:44:33
Re: LOX/CO2-Diborane rocket for reusable landing boats
There are three issues with the use of borane as a propellant that come to mind:
1) Boron is relatively toxic; ingestion of more than 1 g of Boron per day will probably eventually result in adverse health effects over the long run. Not nearly as bad as lithium but it's something to consider.
2) Boron is relatively uncommon, although more than I expected before I looked this up. Production is about 550 million kg per year. For comparison, the Saturn V first stage had about 600,000 kg of RP-1. 600,000 kg of Diborane would have 470,000 kg of Boron, so the price could be expected to rise significantly if we use Boron in rocket fuel.
3) Diborane is nasty. I believe it's hypergolic in air and is hard to handle.
Certainly not impossible, and it would be great to have a high-Isp/high-density propellant. Really wonderful. I know that some experiments were done with Borane in the US in the 50s (scraps of information here), and it was found to be undesirable, but maybe that wouldn't be the case if we went back and looked at it. Who knows!
-Josh
Offline
Like button can go here
#3 2014-04-14 14:33:11
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,134
- Website
Re: LOX/CO2-Diborane rocket for reusable landing boats
Yeah, borane and borax are not the same. There used to be a laundry detergent with borax in it, so that form of boron is fairly plentiful. I think it was "Twenty-Mule Team", and I think Ronald Reagan was its TV pitch man. This was 1955 stuff.
I think Quaoar likes my Mars landing boat designs. He's looking for better propellants for it. I did take a look over in the other thread at that organo-metallic stuff with the lithium in it. Isp-wise, it does look pretty good. Lithium-poisoning-wise, maybe not so good. Peroxide stability-wise, not good at all.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#4 2014-04-20 10:46:22
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: LOX/CO2-Diborane rocket for reusable landing boats
Yeah, borane and borax are not the same. There used to be a laundry detergent with borax in it, so that form of boron is fairly plentiful. I think it was "Twenty-Mule Team", and I think Ronald Reagan was its TV pitch man. This was 1955 stuff.
I know diborane is toxic. I propose it as a possible propellant because is the only I know that can react with atmospheric CO2. Storing atmpspheric CO2 and use it directly as an oxidizer may be a very practical and reliable form of ISRU, because it needs few energy and few time than chemical processing. Using it on your landing boat will save almost 16 tons that can be used for payload.
I think Quaoar likes my Mars landing boat designs. He's looking for better propellants for it. I did take a look over in the other thread at that organo-metallic stuff with the lithium in it. Isp-wise, it does look pretty good. Lithium-poisoning-wise, maybe not so good. Peroxide stability-wise, not good at all.
GW
Yes, I like very much your landing boat. I'm only concerned about storing LH2 for long periods on Mars surface: multi-layered insulation doesn't work in atmosphere and we have to use other matherials like aerogel. Is it possible, with some kind of very efficient active cooling, to keep your landing boat on Mars for months without losing ascent propellant for boil-off?
Happy Easter for all!
Last edited by Quaoar (2014-04-20 10:47:08)
Offline
Like button can go here
#5 2014-04-20 11:05:34
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: LOX/CO2-Diborane rocket for reusable landing boats
Sure using CO2 would be less energy intensive since there is no electrolysis or RGWS used to create the LOX that we would use for Methane or other such fuels. Then if we need to use a canted hypergolic fuel such as in the Draco design then we have more problems for when we create fuels from insitu than we first had thought. In either case we need power to make this all happen. That said we should bump the topic of power and which forms can we ship and or create on mars for fuel creation.
Offline
Like button can go here
#6 2014-04-20 14:24:59
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: LOX/CO2-Diborane rocket for reusable landing boats
Sure using CO2 would be less energy intensive since there is no electrolysis or RGWS used to create the LOX that we would use for Methane or other such fuels. Then if we need to use a canted hypergolic fuel such as in the Draco design then we have more problems for when we create fuels from insitu than we first had thought. In either case we need power to make this all happen. That said we should bump the topic of power and which forms can we ship and or create on mars for fuel creation.
My hypothetical diborane rockets use LOX for descent and CO2 only for ascent: diborane is very reactive with LOX and probably ignition will be easy. If not we can start it with hybergolic, like in Russian school rockets.
Last edited by Quaoar (2014-04-20 14:47:25)
Offline
Like button can go here
#7 2014-04-20 15:42:27
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: LOX/CO2-Diborane rocket for reusable landing boats
Here is a couple of older threads for the use of CO2 Diborane plus other fuels to use with a possible insitu refueling.
Silane Hoppers - Use the CO2 man...
or send the fuel from the moon or other locations that we can get it from
Developing the cis-Lunar economy and infrastructure
While the document Powdered Magnesium—Carbon Dioxide Rocket Combustion Technology for In Situ Mars Propulsion might seem off topic it contains the research for Diborane B2H6 analysis. The Boron would be locked up as an oxide such as it is here on earth B2O3.
A Baseline two-stage ascent vehicle performance is also discussed in the article.
http://www.novasep.com/technologies/Borane.asp
There is a nice mineral table of mars surface contained in this one:
Hybrid Rocket Propulsion and In-Situ Propellant Production for Future Mars Missions
http://svn.developspace.net/svn/minmars … n-situ.pdf
Nice rock table in this one
http://ssi.org/2010/SM14-proceedings/In … rovich.pdf
Last edited by SpaceNut (2014-04-20 17:56:59)
Offline
Like button can go here
#8 2014-04-20 19:41:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: LOX/CO2-Diborane rocket for reusable landing boats
Continuing my search for more information I chanced across Pros and cons of Diborane fuel which would suggest that a surface furnace fuel it would be good for but less so for rocket use.
So where would we get boron baring minerals
http://pediaview.com/openpedia/Borate_mineral
There are over 100 different borate minerals.[2][3] Borate minerals include:
◦Kernite Na2B4O6(OH)3·3(H2O)
◦Borax Na2B4O5(OH)4·8(H2O)
◦Ulexite NaCaB5O6(OH)6·5(H2O)
◦Colemanite CaB3O4(OH)3·(H2O)
◦Boracite Mg3B7O13Cl
Offline
Like button can go here
#9 2021-04-16 21:07:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: LOX/CO2-Diborane rocket for reusable landing boats
bump
Offline
Like button can go here