New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
Pages: 1
#1 2014-03-08 20:24:09
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Methane Magic
Were people aware Musk had come out so clearly in favour of a methane economy for Mars?
http://www.gizmag.com/spacex-methane-mars/25158/
I have always been a fan of methane, so glad to see Musk agrees!
What got me researching this was wondering how easy it would be to use ISRU to create methane manufacture on Mars. Any thoughts on that? Because if the answer is "relatively easy" then I think methane is the way to go for Rover power: set up way stations producing methane which the Rovers can then tap into to.
Any thoughts?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#2 2014-03-08 21:05:03
Re: Methane Magic
Manufacturing methane is fairly easy, and a process to do so was detailed by Dr. Zubrin in The Case For Mars. The raw materials are water and carbon dioxide, and the setup is mostly steel pipes. I believe a ruthenium catalyst (probably imported from earth for a while, since ruthenium is a hard to find, rare metal) is required.
Electrical to chemical energy conversion efficiency is 35-40 percent, because the sabatier reaction wastes a lot of hydrogen.
Methane-oxygen is a high density, simple energy storage method. But it's not energy efficient and it does necessitate cryogenic cooling.
I never understood the notion of characterizing an economy based on its preferred method of energy storage. Surely there are many much more significant ways to characterize a society?
-Josh
Offline
Like button can go here
#3 2014-03-09 18:21:14
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Methane Magic
Manufacturing methane is fairly easy, and a process to do so was detailed by Dr. Zubrin in The Case For Mars. The raw materials are water and carbon dioxide, and the setup is mostly steel pipes. I believe a ruthenium catalyst (probably imported from earth for a while, since ruthenium is a hard to find, rare metal) is required.
Electrical to chemical energy conversion efficiency is 35-40 percent, because the sabatier reaction wastes a lot of hydrogen.
Methane-oxygen is a high density, simple energy storage method. But it's not energy efficient and it does necessitate cryogenic cooling.
I never understood the notion of characterizing an economy based on its preferred method of energy storage. Surely there are many much more significant ways to characterize a society?
Thanks Josh.
I suppose my point is that if you distribute the methane production it might not be efficient but it becomes very practical for Rover use.
Well I guess energy is a pretty fundamental element in any economy, so it is a bit of a marker, but I take your point that it's certainly not the whole story.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#4 2014-03-09 22:32:08
Re: Methane Magic
Certainly, methane is a strong candidate for rover fuel. Although it really depends if the rover is manned or not. An unmanned rover could be allowed to slow or even stop at night if it's travelling from point A to point B.
-Josh
Offline
Like button can go here
#5 2014-03-10 19:24:56
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Finding the energy to make methane is the problem. When we look at Earth having 1367 W/m2 while Mars is at about half of what we see in summer and less under atmospheric conditions but winter seems to drop down to only 1/4th. Table 2.2-1. on page 6 gets the change in location
http://spaceclimate.net/Mars.solar.2005.pdf
Then for man to survive we need power to make water, air and heat on top of the initial values being recieved being very low from solar means we are trying to over come quite a deficit.
Storage of the energy for later use will be of great importance and methane is just one of the many choices that we can make.
Offline
Like button can go here
#6 2014-03-10 19:56:40
Re: Methane Magic
It really depends, of course, just how long we want to store the energy for. For example, rather than making methane it would be much more efficient to use sunlight to heat something up and convert its stored thermal energy to electrical energy at some later date. Large amounts of energy or longer periods of time would make this impractical and thus make turning to methlox (or some other kind of chemical propulsion) quite vital. Effective batteries are unfortunately rather difficult to make.
-Josh
Offline
Like button can go here
#7 2014-03-11 10:03:54
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,110
- Website
Re: Methane Magic
Mars has a neutral atmosphere, not an oxidizing atmosphere. So it's not about the fuel the way it is here, it's really about the oxidizer. That will be the biggest, heaviest thing you have to carry, by far, on Mars.
If it's oxygen, you're either looking at cryogenic LOX or bottled gas. Of the cryogenics, handling LOX isn't too bad. Breathing oxygen systems have long used a Dewar of LOX as a source. It's easier to get uncontaminated O2 that way, than it is by the O2-producing emergency solid propellants. Those often cause problems. I'm surprised they do it that way, but they do.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#8 2014-03-11 15:00:41
Re: Methane Magic
Again, it depends on the application. Compressed oxygen gas might be fine for the dust storm reserve, but not for transportation. Perhaps there is a liquid in which oxygen is very soluble (Would it be possible to make an Oxygen clathrate in Sulfur?)
This is an interesting and new problem, and it's one that would benefit from significant amounts of research.
-Josh
Offline
Like button can go here
#9 2014-04-13 09:49:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Sounds to me that the navy is creating a system to make heavy fuels... which would be something mars could use as well....
(Newser) – In the not-too-distant future, the US Navy could be getting some fuel from the very seas it sails on. That's thanks to Navy researchers who say they've figured out a way to convert seawater into jet fuel, the Huffington Post reports. Experts have been working on the idea for almost a decade, Discover notes; it could be commercially viable within 10 years, the Navy says. Right now, however, researchers are showing off the technique using a model plane.
It works by pulling carbon dioxide and hydrogen from water using a catalytic converter, Discover explains. Those gases are turned into a liquid hydrocarbon fuel that could, experts hope, power both planes and ships, AFP reports. "We don't necessarily go to a gas station to get our fuel," Vice Admiral Philip Cullom tells AFP. "Our gas station comes to us in terms of an oiler, a replenishment ship. Developing a game-changing technology like this, seawater to fuel, really is something that reinvents a lot of the way we can do business." (In other fuel news, scientists have figured out how to make ethanol without corn.)
http://www.huffingtonpost.com/2014/04/0 … 13822.html
http://blogs.discovermagazine.com/d-bri … 0cAV8dRFh4
Offline
Like button can go here
#10 2014-04-13 12:02:03
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,299
- Website
Re: Methane Magic
Fighter jets burn a grade of kerosene. It isn't methane, and isn't ethanol. Members of the Mars Society have been working for a long time on how to make fuel from stuff we find on Mars, starting with water and CO2 and using power from a nuclear reactor. The Navy noticed, fuel for their fighter jets is the same as RP-1, just less refined and different additives. They've been working to produce jet fuel using power from the carrier's nuclear reactor. Mars technology applied to Earth before we even leave. This also means practical application of technology we need for Mars.
METAMARS was a project of Pioneer Astronautics in 2001, Robert Zubrin's company. Principle investigator was Tony Muscatello. Part of the pitch was this technology could be used on Earth. I guess the Navy jumped on it.
Offline
Like button can go here
#11 2014-04-13 14:02:58
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Aromatic fuels was talked of here in Using Aromatic Hydrocarbons for Mars Mission
Like you noted practical applications of R&D that can be directly applied for mars from the start.
It was along time ago that over on MarsDrive the group contest had talked about using such fuels as insitu produced and here is one of the designs. http://aftercolumbia.tripod.com/final0710.pdf
Last edited by SpaceNut (2014-04-13 14:17:21)
Offline
Like button can go here
#12 2014-04-13 21:03:51
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,110
- Website
Re: Methane Magic
Jet-A is the same product from the oil companies as JP-5, just bought to different specs. RP-1 and K-1 kerosene (for stoves and heaters) is almost the same product, differing mostly in cleanliness and additive packages.
Jet-B and JP-4 are also the same product from the oil companies, bought to different specs. The difference is that these are "wide-cut" products, meaning a wider mix of compounds with a wider range of molecular weights and boiling points than the other kerosenes. A half-and-half mix of gasoline with Jet-A is a pretty good approximation to Jet-B.
A diesel engine can be made to run on almost any fuel, as long as it has a low autoignition temperature with air, which means low octane and high cetane numbers. Diesels would run quite well on natural (or casing head, or "drip gas") gasoline, which is typically about 30 motor octane. Spark ignition requires high autoignition, which is high octane / low cetane.
Turbines don't give a tinker's damn about octane or cetane, they can be made to run on just about anything. That would include gasoline, jet fuel, diesel fuel, ethanol, methane, and hydrogen. The mods are to the fuel injection rig, not the engine. You have to be able to meter and finely-spray it, nothing more.
About the only difference between biodiesel and no. 2 petroleum diesel is heating value (just under 18,000 BTU/lb biodiesel vs almost 19,000 BTU/lb for petroleum diesel), and freezepoint (biodiesel without additives freezes about +29 F). Otherwise, biodiesel works as a drop-in fuel in diesel engines. No mods required at all. But, while biodiesel is made from vegetable oil in many cases, it is not vegetable oil. Vegetable oil is a diesel fuel, but not a drop-in fuel.
I used 20 to 30% biodiesel blends in jet fuel about 20 years before interest in this subject became widespread. It's a drop-in fuel in turbine engines. The differences are freezepoint nearer -20 F than -58 or -60 F, and a heating value loss so small you cannot find it in test data on the dynamometer or in flight. I did find a good trace anti-freeze agent that worked to -68 F or lower, in a B-30 blend with Jet-A, but that's another story.
Ethanol performance in turbines is lower and proportional to volumetric heating value. Ethanol doesn't work in diesels, because it has super-high octane and super-crappy low cetane. In spark ignition engines, you can blend it with gasoline up to about 35% ethanol, and not do any modifications at all. Beyond E-42, there are three required modifications, which if not done right, results in poor characteristics.
I have done all these things. For many years now.
The trouble on Mars isn't the fuel, it's the oxidizer. None is in the atmosphere. That's totally unlike here.
GW
Last edited by GW Johnson (2014-04-13 21:08:11)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#13 2014-04-14 21:04:41
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
The oxidizer is hidden in plain sight as a variety of compounds...
http://www.jpl.nasa.gov/news/news.php?release=2012-380
complex chemistry within the Martian soil. Water and sulfur and chlorine-containing substances, among other ingredients,
http://www.jpl.nasa.gov/spaceimages/det … d=pia16575
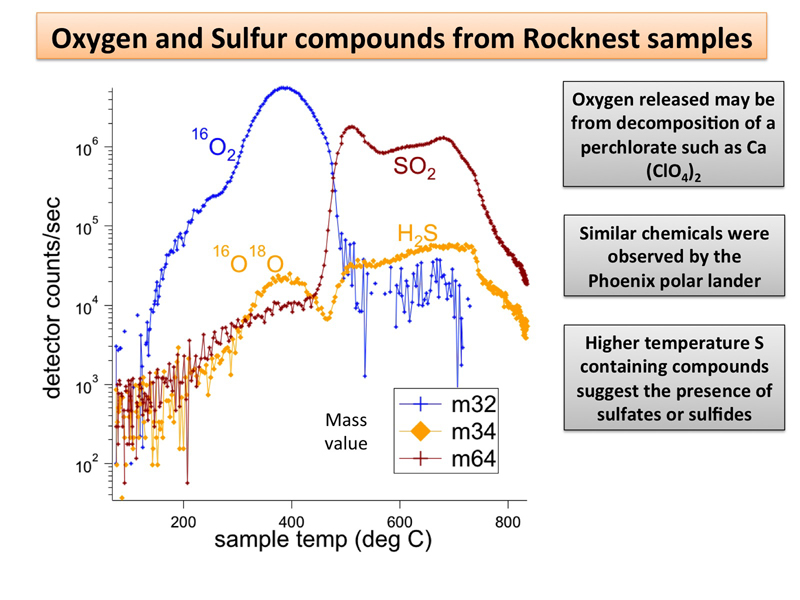
Offline
Like button can go here
#14 2014-04-20 21:02:18
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Something else to consider when we try and use CO2 from the atmosphere is to Utilize trash as feedstock (solids, plastics, etc.) to make water and methane fuel as the Pyrolysis processing of plastic trash and crew waste with in-situ oxygen can make methane.
http://pyrolysisofplastic.blogspot.com/
An Improved Pyrolyzer for Solid Waste Resource Recovery in Space
http://www.susana.org/docs_ccbk/susana_ … herzen.pdf
We can also use Bacteria for the waste to methane process as well Prototype to Recycle Human Waste into Energy & Water
Offline
Like button can go here
#15 2014-05-19 18:38:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Earth organisms survive under Martian conditions
The species were tested for their ability to withstand Martian freeze-thaw cycles that are below the organisms' ideal growth temperatures: 37 degrees Celsius (98.6 degrees Fahrenheit) for M. formicicum and 55 degrees Celsius (131 degrees Fahrenheit) for M. wolfeii.
Offline
Like button can go here
#16 2014-05-21 20:33:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
Chilled Microbes Responsible for Mars Methane Mystery?
One of the most vexing (and exciting) puzzles on Mars is that of the detection of methane in the red planet’s atmosphere. As methane breaks down quickly when exposed to ultraviolet light from the sun, there must be a production mechanism below the Martian surface replenishing the organic gas as detected by Mars orbiters
and astronomical observations from Earth.But is that mechanism geological or biological in origin? In ongoing research at the University of Arkansas, there’s a focus on the latter.
During experiments on specific types of hardy microorganisms that produce methane, known as methanogens, researchers have been able to identify two species of the single-celled bacteria that could set up home under the frigid Mars regolith.
Although these microbes live on Earth, their life-cycle is very alien to our everyday experience. Methanogens do not require sunlight, oxygen or organic compounds to live, instead they metabolize hydrogen (for their energy source) and carbon dioxide (for their carbon source). As a waste product, these microbes generate methane. Methanogens are commonly found in the guts of cows and other animals and happily live in sub-surface environments.
Could extraterrestrial methanogen-like life be eking out an existence on Mars?
The survival of these two methanogen species exposed to long-term freeze/thaw cycles suggests methanogens could potentially inhabit the subsurface of Mars,” said Mickol. The two methanogens were selected as one is a hyperthermophile and the other is a thermophile, meaning they survive in extremely hot environments
(such as geothermal vents) and warm environments, respectively.During the experiments, the methanogens weren’t exactly happy campers, but they survived.
“The low temperature on Mars inhibited their growth, but they survived,” said Mickol. “Once they got back to a warm temperature, they were able to grow and metabolize again. I wanted to see if these cold temperatures would kill them, or if they were able to survive and adapt.”
Although this research doesn’t identify how life may have been sparked on Mars, nor does it suggest that there is life on Mars, it does provide a clue how life can find a way even in the most extreme environments and identifies a possible methane production mechanism on the red planet.
Offline
Like button can go here
#17 2021-01-15 16:10:21
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,524
Re: Methane Magic
This topic contains the word "methane" and it is the oldest topic that does, so I decided to bring it back into view with this update ...
The update itself is from FriendOfQuark:
Don't know if you are still in the thread about converting wind to methane. I saw this article and thought it might add something to the discussion. Zinc based catalyst allows for one step process more efficient than sabatier process - https://www.inceptivemind.com/new-way-c … H4f3-hk-Kc
(th)
Offline
Like button can go here
#18 2021-01-15 18:13:52
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,939
Re: Methane Magic
The changing of the catalyst is part of the cost plus efficiency game for the conversion of co2 and a source of hydrogen which is water. We may gain on one front but maybe not on the other as the energy to get water is still an unknown as even when we send rovers to mars one site may not be much of an indicator as to what maybe possible at another.
The chamber where the reaction takes place will still need to be brought up to reaction temperature in order to get the reaction going with the flow and pressure required to mix ratio where it forms in the heat.
The only difference is at what temperature and what ratios with the catalyst produces the amount of methane that comes out.
Offline
Like button can go here
Pages: 1