New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2014-01-15 10:31:00
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Best propulsion for a long range rover
Mars first manned mission will be wisely centered on exploration and prospecion for a reliable water source for the first stable base, like buried glaciers in low latitudes. So, a couple of powerful rovers with drill equipment, large pressurized habitat ad a big reserve of consumables for a long range cruise (3000 km or more) will be a very valuable tool.
I'm not an expert. I can imagine only theese options:
1) Rechargable batteries/fuel cell and orientable solar array
2) Big deployable solar panel carpet + recargable battery/fuel cells (sun peek stay and navigation douring dawn)
3) Sterling Dinamic Isotope RTG + rechargable batteries/fuel cell
4) Fuel cell + big reserve of fuel
5) internal combustion engine + big reserve of fuel
6) 100 KW nuclear reactor towed with a 50-100 m cable
Wich may be the best option?
Offline
Like button can go here
#2 2014-03-07 10:44:51
Re: Best propulsion for a long range rover
I would say that for a mobile rover, the nuclear reactor and the RTG are out. We can't get the radioisotopes for the RTG, and it's simply not practical to carry a nuclear reactor around all the time. To me it's between solar-battery and carried fuel. Solar-battery is more efficient, but chemical fuels are much lighter and easier to deal with (That's why we use them on Earth!) Methane fuel cells exist, too. The technology is not quite mature yet but I could see that as a strong contender for mobile vehicles (both on Earth and on Mars-- Fuel cells generally achieve pretty high efficiencies.
-Josh
Offline
Like button can go here
#3 2014-03-07 11:05:56
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,133
- Website
Re: Best propulsion for a long range rover
I'm not up to date anymore on fuel cell technologies, but the ones they talked about for cars here were methanol-air fuel cells. There was a rectifier stage that converted methanol to hydrogen and CO2, releasing the CO2 to the air. The actual fuel cell was hydrogen-oxygen, just diluted with nitrogen. Methanol is made most cheaply here from natural gas.
I'd guess the methane-oxygen fuel cell would be similar, leading to the same basic hydrogen-oxygen reaction basis.
But if it was me, I'd just use hydrogen-oxygen as the type that has the most history behind it, for reliability's sake. That gets the technology development out of your program (if you do technology development instead of just basic shakedown of new hardware, you won't ever really fly).
I'd ship water and a solar electrolysis rig to Mars as a stationary plant, and I'd run the rover from stored compressed gaseous oxygen and hydrogen in ordinary bottles. Do the compression at the stationary plant, and just load bottles on the rover for the trips. You could have a solar panel and a battery on the rover as a low-power emergency back-up.
I'd restrict rover trips to a few 100's of km, and do the longer excursions as suborbital flights (with a reusable lander, naturally). You send enough water to supply your basic mission objectives. If you successfully find more water, just process it to supply more explorations beyond your basic objectives. If your lander uses hydrogen-oxygen engines, then so much the better. Add only a liquifaction plant, and you get to fly extra suborbital trips in it.
Basic water, solar electrolysis plant, compressed gas bottle-loading plant, gas liquifaction plant, cryo-tank storage vessels. A habitat building of some kind, plus space inside the landers to live. Rovers with drill rigs. Add a front end loader to experiment with construction techniques. Sounds like a first base to me, to be established on the first (and possibly only) government-sponsored manned mission to Mars.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#4 2014-03-07 21:37:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: Best propulsion for a long range rover
The size of the rover plus fuel tanks is the limitations of the farthest that it can travel minus ideling since power for the rover under a fueled system means that we will need even bigger tanks to make it possible to travel what we would want from a single point source of fuel.
Since we will not have that sort of multi fueling stations on mars for quite some time we will need to think smaller scale exploration instead until we can either build insitu station from mars or land then from earth.
Offline
Like button can go here
#5 2014-03-07 22:47:41
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Ballard developed hydrogen fuel cells. A few other companies worked on it. It used a proton transport membrane, with extremely thin layer of platinum on the polymer membrane. Hydrogen on one side of the membrane, air on the other. For hydrogen storage, use a pressurized hydrogen tank filled with carbon fibre batting. You can use graphite, but carbon nanofibres are lighter with more surface area. Hydrogen bonds to carbon, but when pressure is reduced the carbon releases to become gas. This triples the mass of hydrogen you can store in a tank with the same pressure. I proposed another tweak: composite pressure vessel. Some argued hydrogen cannot be stored in a composite vessel, but I came up with an idea that should work. A bladder of aluminized Mylar. The aluminum clogs the pores in the polymer, and Mylar can withstand temperatures down to -60°C. Basically, hydrogen is contained with metal, within the aluminum coating. This would allow a fibreglass outer shell to contain the force of pressure. On Mars, you would use PCTFE polymer film instead of Mylar, because PCTFE becomes embrittled at -240°C instead of -60°C. But back to Earth. Ford paid Ballard to develop a hydrogen fuel cell for one of their cars. Then as a test, they drove the car from Vancouver to Calgary. It didn't make it. When the car drove into the Rocky mountains, the high altitude resulted in reduced partial pressure of oxygen. That caused the hydrogen fuel call to "stall". Exact details were not released, but the fuel cell didn't function sufficiently for the car to drive. Ford then gave up, and without Ford's money, Ballard folded. Oops.
But that's hydrogen fuel cell. A vehicle on Mars would have to carry oxidiser. Robert Zubrin suggested internal combustion using methane and oxygen. Cars today are fuelled with natural gas, and that's mostly methane. So we know what a vehicle storage tank is like. You could argue gas turbine like an Abrams tank instead of a piston engine, but is that really more reliable? Why not just a hybrid vehicle? Today hybrids run the engine at optimal performance for maximum fuel efficiency to generate electricity. Then store electricity in a battery, and run the vehicle with electric motors. Very fuel efficient. Do the same on Mars.
GW Johnson: Which would be more efficient? A pressurized oxygen tank similar to those used by welders? Or a tank of liquid N2O4? That's the oxidizer used by RCS rocket engines on the Shuttle. Liquid at room temperature. Or MON-3, which is mostly N2O4, just less pure. MON = Mixed Oxides of Nitrogen, and MON-3 is 3% nitric oxide. MON-3 is used by Cygnus.
Last edited by RobertDyck (2014-03-08 08:22:19)
Offline
Like button can go here
#6 2014-03-08 04:32:06
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Best propulsion for a long range rover
To GW: it's possible to bring on mars surface sam kind of light weight cryogenic hardware for H2 liquefaction and active cooling?
To Robert Dycke: an hybrid vehicle with LOX-LCH4 internal combustion engine plus bacteries electric motors and solar array seems a very interesting and intrinsecally safe option. Probably it can have an exploration range up to 1000-2000 km.
Offline
Like button can go here
#7 2014-03-08 10:22:18
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,133
- Website
Re: Best propulsion for a long range rover
IC or turbine engines with fuel and oxygen are going to suffer really high combustion chamber gas temperatures. Here in the air, nitrogen is a huge dilution gas, limiting flame temperatures to about 4000 F max. Fuel-oxygen flame temperatures are usually in the 5000-6000 F range. It's a very difficult engine to build.
It was tried as diesel-hydrogen peroxide in submarines on multiple occasions, but never very successfully. Overheat problems and peroxide instability ended that stuff, more so the unstable peroxide explosions inside a pressure hull. But engine overheat was a very, very serious problem, even with massive amounts of seawater for cooling available.
I have no problem with LOX-LCH4 for some sort of engine, but you will have one huge (as yet unknown) cooling system, or you will have a huge supply of inert diluent gas, or both. You can't feed back the exhaust gases to use as diluent gases without cooling them first.
This is a fundamental area of new technology that is not developed yet. You should not make your mission contingent on developing new technologies, or you will not ever fly. That's been the experience history. The trick with really flying is to use existing technology, so that all you have to do is prove-out your specific hardware designs. That way, you already know that the technology underlying them already works. That's a huge, huge difference.
Liquifying hydrogen is now a very well-known technology here on Earth, unlike 1960. I suspect (engineering experience and intuition) that coming up with hydrogen-liquifying equipment suitable for robot operation on Mars would be easier, faster, and cheaper than coming up with a new IC or turbine engine technology capable of handling fuel-oxygen flame temperatures.
But at least folks are looking at these ideas. That's good.
The hydrogen-oxygen fuel cells used since Gemini in space are just that: bottled gas feeds to the unit. Those work fine in vacuum. You could just as easily use liquified gas sources, and they did on Apollo, at least for the oxygen.
I like the aluminized plastic film idea for hydrogen cryo-tanks. That should stop the gross leaks through porous composite materials. But, hydrogen percolates right through all materials at a slow rate. Even steel. It's a molecular-scale diffusion thing through the pore spaces between atoms. A welding gas bottle of hydrogen will lose around 30% of its pressure in a few weeks.
As for liquid versus compressed gas storage, it's a trade. Bottles are heavy. So is a liquifaction plant. Take your pick.
GW
Last edited by GW Johnson (2014-03-08 10:29:16)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#8 2014-03-08 16:22:12
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Best propulsion for a long range rover
Liquifying hydrogen is now a very well-known technology here on Earth, unlike 1960. I suspect (engineering experience and intuition) that coming up with hydrogen-liquifying equipment suitable for robot operation on Mars would be easier, faster, and cheaper than coming up with a new IC or turbine engine technology capable of handling fuel-oxygen flame temperatures.
But at least folks are looking at these ideas. That's good.
If liquifying hydrogen is quite simple, why limiting it only for rover fuel cells?
Why not reconsidering the whole mission, sending an unmanned lander tanker with a stak of empty modular propellant depot?
The lander tanker can land on summers at high norten latitudes, where there are well known ice packs, melt the ice, electrolize water and bring LOX and LH2 to the orbital propellant depot, to refuel an orbit to orbit manned trasfer vehicle.

Last edited by Quaoar (2014-03-08 16:26:10)
Offline
Like button can go here
#9 2014-03-08 19:43:16
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Offline
Like button can go here
#10 2014-03-09 10:44:49
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Best propulsion for a long range rover
Even in Elysium Planitia (5°N 150°E) there is probably a very big buried glacier:

It's only 5° above the equator and it may be a perfect landing site for a manned mission, but I don't know how difficoult may be for an unmanned lander-tanker to drill and extract water. It may be simpler to land it northen on a well known ice pack, so it will have only to melt ice, electrolize water, liquefie LOX and LH2 and take off to fill orbital depot.
Last edited by Quaoar (2014-03-09 10:47:21)
Offline
Like button can go here
#11 2014-03-09 10:57:02
- Quaoar
- Member
- Registered: 2013-12-13
- Posts: 665
Re: Best propulsion for a long range rover
The hydrogen-oxygen fuel cells used since Gemini in space are just that: bottled gas feeds to the unit. Those work fine in vacuum. You could just as easily use liquified gas sources, and they did on Apollo, at least for the oxygen.
GW
So we can imagine some sort of hybrid rover, with a dual powered electric motor: H2-O2 (or O2 CH4) fuel cells plus solar arrays and bacteries: when the Sun is high it uses solar arrays saving fuel an when it goes downhill, the wheels recharge the bacteries, like in the hybrid vehicles.
The rove may have also a drilling hardware to find water, so it can exted the exporation range to almost 1000-2000 km.
Offline
Like button can go here
#12 2014-03-09 20:17:06
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,378
Re: Best propulsion for a long range rover
I doubt that water will be found in the manner that we think of water here on earth as it will be frozen to the soil or board in the minneral such that its going to take energy to seperate it out once it is mined.
The electric hybrid engine supplies power to the wheels once it gets to 30 mph as the power to the electric motor gets to great for the batteries to continue to supply direct power. The rover will not be traveling that fast for sure.
Agreed that solar power will aid the distance that a rover will travel but you still need to either find another supply to get water from or turn around and go back to home base.
Offline
Like button can go here
#13 2014-03-09 21:48:04
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
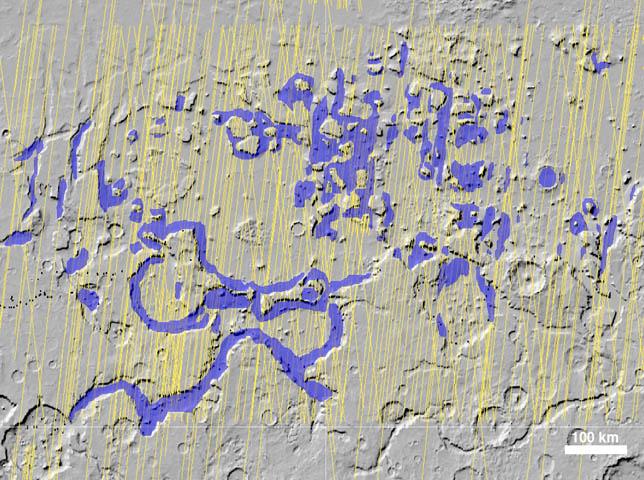
Caption from the SHARAD image above...
The Shallow Radar instrument on NASA's Mars Reconnaissance Orbiter has detected widespread deposits of glacial ice in the mid-latitudes of Mars.
This map of a region known as Deuteronilus Mensae, in the northern hemisphere, shows locations of the detected ice deposits in blue. The yellow lines indicate ground tracks of the radar observations from multiple orbits of the spacecraft.
The ice, up to 1 kilometer (0.6 mile) thick, is found adjacent to steep cliffs and hillsides, where rocky debris from slopes covers and protects the ice from sublimation into the atmosphere.
The base map of this image is shaded relief topography obtained by the Mars Orbiter Laser Altimeter on NASA's Mars Global Surveyor. The image is centered at 42.2 degrees north latitude and 24.7 degrees east longitude. It covers an area 1050 kilometers by 775 kilometers (650 miles by 481 miles).
This means ice. It'll probably be salty, with gravel and dirt, but that can be filtered out. A reverse osmosis filter to desalinate requires a lot of pressure. That will require power. But it is ice.
Water to Arrakis!
Offline
Like button can go here
#14 2014-03-10 00:11:43
Re: Best propulsion for a long range rover
In terms of colonization I still think that the water discovered at Elysium is the most promising source, because it's closer to the relatively abundant solar energy there. Unfortunately the evidence for buried ice is less convincing there, so we can't be sure, but it's certainly possible (if buried under enough regolith). Water is of course the single most important material resource. Energy is equally vital, of course.
When it comes to fuel, oxidizer is the really troubling one. For a rover intended to spend a long time away from base, it's ideal that its propellant be stable at martian external temperatures. Ammonia is a pretty reasonable choice for a fuel, with the added bonuses that it's easy to make and will have other applications. Methane is less good, since it would boil away (Martian temperatures are typically around 230 K or so). Methanol isn't bad, and has the added bonus of being a liquid at room temperature as well as martian temperatures.
However, both of these would need an oxidizer, which is significantly harder to come by. LOX is a sub-martian cryogenic, and peroxides and nitrogen oxides are hard to produce. If they exist naturally on Mars this would be wonderful but cannot be counted upon. Sulfur trioxide is a decent oxidizer, but it doesn't give up very much oxygen per unit mass (Just 20%). Pressurized oxygen might be required.
Chlorates can be prepared fairly simply, so perhaps we should consider using them as oxidizers in mobile chemical fueled platforms.
-Josh
Offline
Like button can go here
#15 2014-03-10 08:51:59
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Well, if you want to talk about new stuff, something that does require research, then I've spoken before about trisilane. It's liquid at room temperature, and any temerature outdoors on Mars. It burns in carbon dioxide, so can breathe Mars air. The issues are that atmosphere will have to be compressed for combustion, and exhaust includes silica scale. You have to design an engine that can withstand silica scale. I said a piston engine would get clogged quickly, so suggested a turbine engine with teflon coated turbine blades, designed to withstand a little out-of-balance. But GW Johnson doesn't think a turbine could work. Someone here suggested external combustion, something like a steam engine, but how do you compress Mars atmosphere?
Silane is made from carbon dioxide and sand. Requires significant energy. After all, all fuel is chemical storage of energy. The issue is it burns. In the atmosphere of Mars, it's a safe to handle as gasoline. However, in oxygen it will spontaneously combust. So you would have to be very careful when taking a rover into a pressurized garage. NASA used monosilane to ignite Shuttle main engines.
Jet engines designed by American military contracts are very sensative. Aircraft carriers often have crews walk the flight deck, a line walk across looking for debris. A single nut ingested into an engine intake can destroy the engine. However, engines for the A-10 Thunderbolt II, commonly called Warthog, are so robust they can withstand small arms fire. And Russian combat jets are designed to land on runways so rough that Americans would think them long decommissioned. Designing a new jet engine that spits out silica scale would require someone with moxy. And intake compression would have to be extreme.
I'm reminded of the story of Canada's RadarSat. The Canadian Space Agency went to NASA proposing a joint project. They wanted a radar satellite that could map sea ice, to tell ships where ice is thin enough, and where water beneath the ice is deep enough. NASA said it couldn't be done. So CSA said fine, we'll do it ourselves. They did it. When it was ready to launch they went back to NASA, proposed they launch it, to pay for the launch America would get 30% of the observation time. NASA took the specifications to the US military who pointed out this is better resolution than anything they have, in fact it can detect the wake of ships at sea. So they said "No". The CSA said fine, that's Ok, we'll just ask the Russians to launch it for us. They'll get the 30% obseration time. Just 24 hours later NASA called back, "When would you like it launched?"
Last edited by RobertDyck (2014-03-10 10:14:19)
Offline
Like button can go here
#16 2014-03-10 09:48:22
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,133
- Website
Re: Best propulsion for a long range rover
The tale about the Canadian RadarSat I find "typical" of big-bureaucracy behavior. It's not just American bureaucracies, and it's not just government organizations. As a rule of thumb, all giant organizations are afflicted to one extent or another. The main symptom to recognize is a level of technical arrogance that is entirely unjustified by the demonstrated level of technical competence.
As for silane turbines: it's reasonably easy to design-out external threats like debris and gunfire. The problem with silane is that the silica slag is generated entirely within the combustor immediately ahead of the sensitive turbine that the combustion gas has to pass through. I'm not saying it's impossible, because you might just do a cyclone separator ahead of the turbine. But it surely won't be easy.
Some other external combustion engine might be done with silane exactly the same way: separate-out the solid slag before the stream reaches the working bits. With piston, it might be easier to handle separations in hot gases a little easier than in turbine. Maybe.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#17 2014-03-10 10:14:00
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Actually, I don't think Teflon can withstand temperatures inside a jet engine. At least not PTFE. However, I saw TV ads for a non-stick frying pan with something called sapphire. Their brand name is FlavorStone. They claim it's a sapphire coating. Synthetic sapphire is crystalline aluminum oxide. That should withstand temperatures in a jet engine.
But I don't think a piston engine would work. Even a tiny bit of silica scale on the inside of a cylinder will block the piston from travelling up and down. Piston rings are designed as springs to scrape against the cylinder wall, they would catch on any silica scale. Not to mention blocking cylinder valves from closing.
A cyclone separator? Working the same way as a modern vacuum cleaner? Great idea! Ok, we have a start.
Offline
Like button can go here
#18 2014-03-10 11:37:17
Re: Best propulsion for a long range rover
My expectation would be that Silane would be fine for use in an external combustion engine, such as a sterling cycle. These are mildly efficient but I've heard that it's tough to get them to generate a lot of torque, which is important for a vehicle. Having said that our rovers don't need to go particularly fast.
The bigger issue with Silane, as I see it, is the difficulty of mannufacturing it. From Wikipedia:
Trisilane is a trace product of the reaction between SiH2 and HCl. This reaction is done using infrared lasers. The laser what set up in a stainless steel cylindrical cell with the laser perpendicular to the mass spectrometer. Trisilane can also be produced by using the Schlesinger process. This process reacts lithium aluminium hydride with octachlorotrisilane in 1-butoxybutane. Trisilane is also yielded by thermal decomposition of monosilane and disilane at higher temperatures as demonstrated by Bowrey, Purnell and Walsh in the sixties.
Disilane is also tough to make. Monosilane isn't too bad but is also not liquid at Martian temperatures. When it comes to fuels, my take is that it's not so important that they have a high energy density so long as they can be made efficiently and cheaply and stored easily.
-Josh
Offline
Like button can go here
#19 2014-03-10 12:39:53
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,133
- Website
Re: Best propulsion for a long range rover
If you can make a fuel such as LH2 or LCH4 on Mars, you can also make LOX. You MUST have water to do that. All you need is a suitable diluent gas to make your engine design practical. There's plenty of CO2 in Mars's "air", all it needs is compression and storage. LCO2 is done fairly readily here.
We've talked about compressing Martian CO2 elsewhere. The first stage needs to be some sort of confined phase-change operation, to get the pressure nearer 1 atm. Once there, conventional compression is quite as easy as it is here. That first compression stage will have huge mass and volume, for a low throughput. That's inherent when your source is first cousin to the vacuum of space (which applies to making LCH4 out of the local "air" whether or not you bring your own hydrogen).
That means this is going to be a batch process, not a continuous-flow thing. You'll not build a silane-CO2 gas turbine feeding directly off of Mars's atmosphere, or any other kind of engine, even if the slag problem has a real solution. You'll instead accumulate fuel, oxidizer, and diluent materials slowly, load them up, and consume them much faster while driving. Turbine, piston, external-combustion, doesn't matter. Just realize that the volume and mass of of the oxidizer and diluent will far exceed that of the fuel. Not any different than here.
That last isn't a real problem. We don't drive around here in cars that carry their own oil refineries, either. But it does impact your planning, and it puts very real constraints on realistic rover ranges. The stationary consumables plant is producing another load, while you are out driving around. But, you must return to refuel. Nothing really hard about it.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#20 2014-03-10 14:27:08
Re: Best propulsion for a long range rover
Producing LOX is easy, sure. Storing and handling it, less so. If the inside of your rover is 300 K and the outside is 230 K, you're gonna need a whole lot of insulation, plus a good refrigerator, to keep your oxygen at 85 K and your methane at 110 K. On Earth, the stoichiometric ratio of air to fuel is 14.7 to 1. This is also a reasonable value for this ratio from a temperature perspective. Of this air, 21% will be oxygen that participates in combustion. Therefore, of 15.7 parts total, there are 3.1 parts of fuel/oxidizer and 12.6 parts of dilutant. That's about 80% dilutant and 20% fuel/oxidizer.
So we would need to take in or store 4 kg of CO2 per kilogram of fuel/oxidizer. This would either pose a significant storage annoyance (high pressure/low temperature/high heat of vaporization if you store it as a solid) or decrease the amount of available energy (compression is necessarily not quite isentropic), plus adds complexity to your rover's engine.
So that's why I'm looking at alternatives. For example, Ammonia does not burn nearly as hot as Methane does. Of course I suppose it could be possible to cycle the cooled exhaust back through the engine a few times (?) which would eliminate the need for external dilutant and simply leave storage annoyances.
I personally would prefer to hold out for fuel that is liquid at 1 atm, at a temperature close to either room temperature or Martian ambient. Plenty of fuels meet this criterion but oxidizers are harder to find. Chlorate salts are fairly easy to make but aren't liquid. Nitrogen oxides are liquid at mild pressures but are very difficult to make. Likewise with peroxide.
-Josh
Offline
Like button can go here
#21 2014-03-10 14:36:40
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Example of silane in air. This is video of Space Shuttle main engine ignition. The bright glowing blobs that spray horizontal beneath the main engines are drops of silane. The igniter is built into the mobile launcher, it sprays silane. As soon as that liquid touches air, it ignites.
http://www.youtube.com/watch?v=urxrOI6-RlE
Ps. It's still awesome seeing the Shuttle launch. Never was able to get to the Cape when it was flying. They kept postponing launches, so it was hard to schedule. The one weekend I did make it, a Shuttle was in space. Full experience at visitor center, and the "Then and Now" tour that included Mercury, Gemini, and Saturn 1 launch facilities. I was there that one Saturday from the minute they opened the visitor center until they closed.
Offline
Like button can go here
#22 2014-03-10 16:38:08
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,133
- Website
Re: Best propulsion for a long range rover
Magnesium burns in CO2, producing MgO slag (usually a liquid), and carbon black (a solid). If you run excess CO2, you can get hot gases, too, mostly just CO2, some CO. Hot gases at high pressure are what is required to do mechanical work in an engine of any kind. Solids and liquids do absolutely nothing for you.
Alone of all the old-time "ramjetters" of 1970-1990's, I used to use magnesium for a ramjet engine igniter. In operation, it looked a lot like the silane "sparklers", except the mag produces a brilliant incandescent sheet of flame, burning in air.
I did it with a solid propellant. It was about 20% AP oxidizer, 20% silicone rubber binder, and 60% fine-ground mag powder. This stuff was utterly reliable at igniting fuel-air reactions, with just about any fuel you can think of. As a solid propellant, it was pretty safe, too. We were stretching things to classify it as a Class 1.3 explosive.
I used it for two decades as tiny cartridge-loaded "motors" that screwed onto bosses on our test articles. But I also used exactly the same propellant as a solid gas generator fed ramjet missile fuel about 1976-1978. It worked just fine in both roles.
Sorry, just adding turbidity to an already murky conversation.
GW
Last edited by GW Johnson (2014-03-10 16:41:20)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#23 2014-03-10 19:03:34
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Pressurized LOX/LCH4 hybrid, with solar assist, is such an obvious choice that we have to talk about other stuff just to have something to say. I do have to emphasize pressurized, you don't want cryogenic liquids on a rover. They won't stay cold, even on Mars. But here on Earth we pressurize them sufficiently to liquefy at room temperature. Takes a lot of pressure, which is why steel tanks are so heavy.
I saw a TV episode of "How It's Made". They showed fibre glass composite tanks for propane. They're lighter than steel, and see-through so you can see how full. Would that work with methane, or does methane require too much pressure?
You raised questions of engine temperature, and combustion diluent. Is that really necessary? Could a robust engine use O2 directly? I'm trying to reduce mass of carried expendables. The more expendables your rover requires, the shorter its range. And couldn't it use radiator cooling? One recent product in stores this year is a home distillation unit. It's made in New Zealand, where home distilling alcoholic beverages is allowed. I noticed home brew stores started to carry "turbo yeast" from New Zealand. It's a combination of distiller's yeast that can withstand 20% alcohol, together with an enzyme that breaks down starch into sugar. And yeast nutrients. The idea is to brew 20% alcohol directly. This would then be distilled, but they didn't carry the distiller because they could get in trouble here. I pointed out that the law in this province doesn't actually say home distilling is illegal. In fact there's nothing in the law that says one way or the other. Lots of laws regulating production of alcoholic beverages for sale, and lots of laws about tax, but nothing about producing alcohol for personal consumption. The provincial government owned monopoly for sale of alcoholic beverages claims it's illegal, and tries to convince the polices to required "offenders" to pay a fine equal to what the taxes and corporate profits would have been if they bought it from them. But there's nothing in the law to authorize that. Someone has to show the guts to stand up to the monopoly. This happened in the 1960s, one businessman took the issue to court. The court ruling was home brewing of beer and wine was allowed. The issue today over home distilling is exactly the same, with the same laws and the same legal arguments. Someone just has to stand up for it. After I explained this, that store now carries the home distillation unit from New Zealand. He advertises it as a "water distiller", but his web site has instructions how to use it to distil alcohol. The reason I raise this, the home distiller doesn't use water to cool condensate. It has a heat sink and fan. So it only requires electricity to distil. It's about the size of a home bread maker, and can distil one gallon at a time. If that unit can do so without coolant, couldn't an engine on Mars?
Offline
Like button can go here
#24 2014-03-10 20:14:23
Re: Best propulsion for a long range rover
I have no issue with pressurized methane, but I'm a bit skeptical that it would be possible to pressurize LOX into liquid form, even at 230 K (In fact, its critical point is at 155 K, 5 MPa, so we would be dealing with a supercritical fluid). That's why I was bringing up other oxidizers, and I still believe that it's an issue that needs to be solved. Gaseous oxygen, at any pressure, is simply not dense enough.
I'm not sure how I feel about pressurized methane, compared to methanol. They are equivalently difficult to make (If anything, methanol is easier and more efficient) and as a plus does not require extreme pressurization. At room temperature, Methanol's vapor pressure is 13 kPa. At 230 K, it is about 100 Pa, which is to say that you wouldn't even need to pressurize it in ambient air.
Even given all this, we don't yet have a satisfactory oxidizer. Again, given the choice between cryogenic liquid oxygen and pressurized gaseous oxygen, I choose neither.
-Josh
Offline
Like button can go here
#25 2014-03-10 22:21:22
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,366
- Website
Re: Best propulsion for a long range rover
Propane is a 3-carbon hydrocarbon: C3H8. This is liquid at very moderate pressure, including the previously mentioned fibreglass.
Here is an interesting paper, at least the abstract sounds interesting:
Direct synthesis of propane/butane from synthesis gas
http://www.sciencedirect.com/science/ar … 910780156X
Last edited by RobertDyck (2014-03-10 22:22:22)
Offline
Like button can go here