New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#51 2012-10-07 10:23:43
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Greenhouse - hydroponics vs soil
If your greenhouse is a tall structure anyway, why not rack your hydroponics vessels above your soil-based agricultural activity. Racks should be easy to build on lower-gravity Mars from most anything. Even lashed bamboo would work, which could be grown there. Including bamboo fiber-based rope for the lashings. A rope-making machine is a very old, very simple technology. You do need to know your knots and your lashings. Not in the usual astronaut training, but maybe it should be.
Think a round foundation in the middle of a small crater or depression, a mushroom-shaped building with a regolith-covered cap for a radiation shield, and clear walls around the "mushroom stem". Surround the thing to the poleward and sunrise-sunset sides with reflectors made of aluminum foil bonded to any convenient substrate (including even permafrosted regolith) as a 3/4 ring solar reflector bringing light inside the building laterally. You can mount your solar thermal and solar PV stuff up on the roof.
Over on the equator-ward side, that's where you set your very large water tanks. They are both your supply and a huge thermal mass. Paint them flat black, and let them be passive solar to the extent possible. It works in my greenhouse here. The other 1/4 ring of reflector surface on the equator-ward side would help amplify the water tank passive solar effect, but also blocks surface access to the building. If you need a roadway in, put it there.
I'm not sure what to make the transparent walls of, but eventually some sort of glass should be possible there, if the right silica sand can be found. I'd recommend a double or even triple wall for the insulative heat trap effect, and for decompression safety.
Comments?
GW
Last edited by GW Johnson (2012-10-07 10:28:15)
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#52 2012-10-08 07:35:48
- Glandu
- Member
- From: France
- Registered: 2011-11-23
- Posts: 106
Re: Greenhouse - hydroponics vs soil
I like the idea of painting in black. Though you need to manufacture carbon black on site for that. Once you can, it seems a very efficient thing to do, and not only for the greenhouse. Anything that needs to be heated, paint in black - like roofs. Any heat you can capture has to be captured. Mars is cold.
For transparent walls, well, I had a quick look to shielded glass, & it seems to be for a much later time(polycarbonate ain't the easiest thing to synthetize, or to process. Plus it has crappy optic properties). Plains, standard glasses, should be enough, anyways, if in thickness.
[i]"I promise not to exclude from consideration any idea based on its source, but to consider ideas across schools and heritages in order to find the ones that best suit the current situation."[/i] (Alistair Cockburn, Oath of Non-Allegiance)
Offline
Like button can go here
#53 2012-10-08 09:03:59
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
At the Mars Society convention in Chicago, I presented a paper on making aluminum from Mars rocks. We've found bytownite on Mars. That dissolves in hydrochloric acid, then neutralize pH by bubbling ammonia through. This reversed the pH for the Bayer processes to extract aluminum hydroxide from bauxite. But instead of sodium hydroxide dissolving aluminum from bauxite, then neutralize with CO2, this process dissolves with hydrochloric acid then neutralizes with ammonia. Then follow the Bayer process: calcinate aluminum hydroxide to get water out, resulting aluminum oxide. Then smelt with electricity.
An interesting thing from this is silica gel will be left behind from bytownite. That gel can be calcinated to form dry silica gel, used as a desiccant. Or calcinate to conclusion to form pure silica (silicon dioxide). So silica for glass could be a by-product of smelting aluminum.
Offline
Like button can go here
#54 2012-10-08 17:16:56
Re: Greenhouse - hydroponics vs soil
Wow robert, I never knew that that could be possible. Perhaps Mars One could use that paper if they start succeeding ![]()
-Koeng
Lets terraform today!
[url=http://www.terraformingforum.com]www.terraformingforum.com[/url]
Offline
Like button can go here
#55 2012-10-08 18:33:51
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
A copy of the PowerPoint is available from my local chapter website here:
http://chapters.marssociety.org/winnipe … ldspar.ppt
Presentation notes are part of it. Save this as a file to your computer, then open with PowerPoint. Turn on "Notes Pages".
Two forms of plagioclase feldspar dissolve in hydrochloric acid: anorthite and bytownite. Any more albite than that results in quartz forming, sealing grains so they won't dissolve.
I believe I wrote it as a printable paper. Send me an email, I'm at my sister's place for Thanksgiving dinner right now.
Last edited by RobertDyck (2012-10-08 18:37:33)
Offline
Like button can go here
#56 2012-10-14 13:22:51
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Greenhouse - hydroponics vs soil
It is very clear viewing RobertDyck's powerpoint that he knows his chemistry and his chemical engineering. If he says we can get aluminum from bytownite on Mars, I believe it! Great news, Robert. Aluminum metal is one very useful material. (What about iron and steel?)
I am no chemist or chemical engineer, the bulk of my training and experience is mechanical/aeronautical. But, in an environmental clean-up situation, I once used ammonia solution (at about 5% strength) to neutralize battery acid (sulphuric acid) that had flowed to inaccessible locations in building wall and truck trailer structures, in a battery acid spill incident. I kept it very dilute, because I was afraid of steam explosions, pouring this stuff directly onto spilled battery acid.
It worked, and far better than the "standard" treatment of spreading powder lime. Plus, the reacted product was just very-slightly-salty water, which we could dilute further and just send down the storm sewer. That clean-up effort was actually far easier and less labor-intensive that way. Spreading lime makes a horrible mess. Plus, we couldn't effectively get lime into the building wall internal structures where the battery acid had flowed, or down through the truck trailer structures where it got to.
We bought industrial ammonia solution concentrate at 10% strength, and cut it half-and-half with water on the job site. I saw bubble formation, but no violence. Sure were a lot of poison fumes inside that truck trailer, though. Time inside was basically limited to breath-hold times. But we got it cleaned it up. The building was better ventilated.
That was an interesting day.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#57 2012-10-14 19:13:07
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
(What about iron and steel?)
The best ore for iron or steel on Mars are actually the hematite concretions that Opportunity found. Problem is they don't occur everywhere. Just Meridiani Planum and a few other locations. Those concretions (initially called "blueberries") are crystalline iron oxide. They're very hard, embedded within soft sedimentary rock. That means they can be separated by crushing, vibration, and sifting. Once you have pure hematite concretions, crush with a more powerful crushing machine. Crush all the way to fines. Then use the direct iron method. This method of smelting is already in use in some countries.
Traditional smelting starts with coal, which is baked in a "coaking oven" to burn off sulphur. Heating and blowing air through burns sulphur to sulphur dioxide gas, the carbon doesn't burn. However, the heat source is usually burning some coal separately. If the sulphur dioxide gas is vented to the atmosphere, then water in clouds together with UV from sunlight combines with it to form sulphuric acid. When that cloud rains, you get acid rain. But if you send the gas through showers of water, especially with UV lamps, then you convert it to sulphuric acid under controlled conditions. That acid can be sold for industrial purposes. Once you have coke (coal without sulphur) then use that as fuel for the blast furnace. The reason for this is a tiny amount of sulphur will significantly weaken steel. Coke is burned to smelt iron, but it's starved of oxygen. This forces the coke to burn with air to form carbon monoxide. That's key, the carbon monoxide combines with oxygen from iron oxide. The result is carbon dioxide. When oxygen is removed, iron is left. But some of the carbon from coke dissolves in liquid iron. This creates an iron/carbon alloy; that's called steel. Usually there's too much carbon, creating steel that's brittle. This is then taken to a Bessemer Converter which re-melts the steel and blows air through, burning off carbon. It has to be controlled, you don't want to convert iron to rust, and to be steel it requires a certain amount of carbon. Not too hot, and just enough air, for just long enough.
The direct iron method is a single step to make steel. Start with highly pure iron oxide fines, it won't work with anything else. Then add pure carbon monoxide gas mixed with hydrogen gas. The hydrogen also sucks oxygen from iron oxide, but that produces steam which does not dissolve in molten iron. Starting with less CO gas results in less carbon in steel. Hydrogen is more expensive than carbon monoxide, but the balance of the two gasses will determine how much carbon is in the final steel. The real beauty of this system is it works at lower temperature. In fact, it's below the melting temperature of steel. It's usually +900°C, which is low enough that a nuclear reactor can produce that directly. Of course if you do that, then you can't use that heat to generate electricity, it's either used to smelt steel or used to generate electricity, not both at once. One key reason to keep the temperature lower is to ensure steel pipes in the reactor don't melt. Of course if you have to melt the product to convert it from sponge steel to bars or rods, that might defeat the advantage. On the other hand, it could make smelting more efficient. Take the sponge steel out while still hot, then use some electricity method such as inductive heating to melt it just enough to roll out air bubbles and form bars or sheets. Again, some commercial smelters already use this on Earth because it's more energy efficient (read "less expensive"). And again, the catch is you need very good ore for it to work.
Last edited by RobertDyck (2012-12-06 00:44:49)
Offline
Like button can go here
#58 2012-12-02 13:59:31
- Number04
- Member
- From: Calgary Alberta Canada
- Registered: 2002-09-24
- Posts: 162
Re: Greenhouse - hydroponics vs soil
In my mind, soil and aquaponics both have their benefits. Soil is easier to start and maintain. It requires fewer resources to bring from earth. Aquaponics takes up a smaller footprint, supports a full biological cycle and is more versatile.
As Robert said, we need backups, so why not both? This would be for a settlement of course, not a touch-and-go science mission.
If we consider a 2000 calorie per day diet for our colonists, the soil garden should be able to sustain at least that. Staple crops should be planted in soil, wheat, potatoes, beans and the like. If the aquaponics fails, or is under maintenance or they bio cycle collapses, the garden should provide food to stay alive. The aquaponics system has a greater storage capacity. Plants and fish need to be in the system to keep it running. The caloric value of the storage of food should be enough to keep the colony alive if the garden fails. Say, 60 days on 1000 calories a day until the soil crops will provide food after full replanting.
The aquaponics system can be incorporated into the waste cycle. People have mentioned Tilapia as a the fish of choice, but crayfish and other bottom feeders can be used to help breakdown vegetable and animal protein. The aquaponics system can provide fertilizer for the soil system if it’s calibrated to “run rich”.
Yes, it adds a layer of complications to the design, but moving forward with both proposals is more productive than arguing about what’s better.
Offline
Like button can go here
#59 2014-09-09 14:39:34
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
Reading the book "How to Live on Mars" by Robert Zubrin. Copyright is 2008, and I think I've had it that long. Fun book. It goes over the same stuff as "The Case for Mars" but tongue-in-cheek, written as if Mars is already settled and you are a pioneer just arriving.
The chapter "How to Grow Food (That Is Actually Edible)" describes greenhouses. It reads as if Dr. Zubrin read the discussions on this forum. But I have to argue about some of it.
All Martian greenhouses are inflatables, employing polypropylene plastic with an antiultraviolet coating. This film is reinforced by internal Kevlar, Spectra, or Nanectra netting, giving the bulk material a yield strength in the 200,000 psi range.
Dr. Penelope Boston raised the issue of UV durability. She said there isn't any material capable of withstanding the UV of Utah, much less Mars. (MDRS is in Utah.) So I researched the issue, and came back to her the following year with samples. And datasheets for each sample.
Tefzel is transparent, and able to withstand cold, but would become brittle in the deep cold of the coldest winter nights on Mars. Tefzel is a co-polymer of tri-fluoro-ethylene (TFE) with normal ethylene. Ethylene is C2H4, and TFE is very similar but has 3 of the 4 hydrogen atoms replaced by fluorine. Regular Teflon is pure polymerized TFE, known as PTFE. Goretex is also PTFE, but a different brand name by a different company. Tefzel is sold as the premier quality plastic film for greenhouses on Earth. It's the most UV resistant, and strongest, but also most expensive. However, it can't withstand the UV on Mars. And has that problem with deep cold.
Teflon FEP is "Fluorinated Ethylene Propylene"; also a co-polymer, but this one is TFE with fluorinated propylene. Teflon FEP can withstand the cold of winter nights on Mars. More expensive, but has an issue with permeability. That is, oxygen slowly leeks right through it.
PolyChloroTriFluoroEthylene (PCTFE) is another modification of ethylene. Like TFE, but the final hydrogen atom is replaced by one chlorine: C2F3Cl. It doesn't become brittle until -240°C, which is 100°C colder than the coldest temperature of the Mars south pole during winter. So it laughs at cold. It's the most impermeable to water of any polymer known to Man. And very impermeable to oxygen. Although there are polymers more impermeable to oxygen, they all become brittle at -60°C or warmer. Mars gets colder than that at night, even the warmest summer night at the equator. So of the polymers that can withstand the cold of Mars, PCTFE is the most impermeable. In fact, it's so impermeable that you could say air just doesn't go through. And it's the second most transparent to light of any polymer. Only Teflon AF (Amorphous Fluoropolymer) is more transparent, but that one is rigid, almost as hard as glass. Don't expect to make a film out of it. And PCTFE is extremely UV resistant; primarily because it's so transparent that UV just shines right through.
PCTFE used to be sold by 3M under the brand name Kel-F. Today it's sold by Honeywell under the brand name Clarus for the military or aerospace industries, or Aclar for the pharmaceutical industry. Since it's so impermeable to water, they use it for blister packs for pills. A Japanese company sells it under the brand name Neoflon, but they use that name for all fluoropolymers they make, so they further qualify it as Neoflon PCTFE.
I also argue to use fibreglass gauze thermally bonded to the fluoropolymer. "Thermally bonded" means press the gauze against the film, and heat it so it melts into the surface of the plastic film. Fibreglass not only reinforces the film, it acts as rip-stop. And fibreglass is transparent. This would have to be further reinforced with straps. This design is for a science mission, a production greenhouse for a settlement would have to be larger. I suppose a 50 foot diameter greenhouse would require the net he described. But I still argue for fibreglass reinforced PCTFE.
The film requires not only UV resistance, but UV blocking to ensure plants don't get sunburn. And IR reflection for radiant heat control. NASA already has a coating that does all this; they apply it to all spacecraft and space station windows, as well as spacesuit helmet visors. It's called "Spectrally selective". It's incredibly thin layers of metal applied by vacuum deposition. It's gold, nickel, and silver oxide. Only silver is oxidized. This has already been commercialized, used for commercial building windows; brand names "Heat Mirror" and "Low e". However, the commercial versions only use silver oxide to reflect IR; they don't bother with gold or nickel for UV protection.
I also argue for inflatables for a greenhouse transported from Earth. To make a greenhouse from in-situ materials, it's easier to use glass. Just normal glass. You would still require the spectrally selective coating. But glass can be made more easily and with less energy than a fluoropolymer. And any fluorine ore is quite rare. It's probably on Mars, but no Mars rover or lander has yet found any. Probably just as rare on Mars as on Earth.
Robert Zubrin's book says only a Moon colony would use glass. But why not Mars? The book raises the issue of material fatigue due to prolonged thermal stress; the Moon gets very hot during the 14-days of sunlight, then very cold during 14-days of dark. But Mars has a length of day practically the same as Earth. And although it gets cold at night, it doesn't get down to -123°C at the equator every night. The Moon does. Earth has winter and day/night, yet our glass windows don't explode. So just use glass on Mars. Yes, it's heavy. But when you make it from local materials, weight isn't much of an issue.
Last edited by RobertDyck (2014-09-10 19:39:37)
Offline
Like button can go here
#60 2014-09-10 09:53:32
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,174
- Website
Re: Greenhouse - hydroponics vs soil
I'm not sure an inflatable greenhouse brought from Earth will ever be practical, precisely because of the cold, the harsh UV, and the pressure differential effects. All the good plastics we have , have serious shortfalls for that environment. In the post just above, RobertDyck turns to advocating glass in a greenhouse built from in-situ materials. That's my favorite concept, too, and it has been for a while (see also post #51 above, from 2012).
I had sort-of fleshed this concept out in a posting on my "exrocketman" site, as a mushroom-shaped masonry building held in place against internal pressures by dead-load regolith on its roof. There is a multi-layer glass panel-between-columns ringwall around the perimeter that lets in light with reduced UV, and keeps the air and heat in. So you get the reduced UV necessary for agriculture inside, and the added benefit of radiation shielding from your roof. All we need is a concrete substitute that works in the cold near-vacuum that is the atmosphere of Mars, and we can build this thing out of mined local rock. Or send framing steel from Earth. Or both.
Y'all might enjoy looking at my old posting. It's something we could build with front-end loaders and a glass-making facility suitable for Mars. And it would work on the moon, too. http://exrocketman.blogspot.com. Use the navigation tool on the left. Go to "2013", then "January", then "Aboveground Mars Houses", posted 1-26-13.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#61 2014-09-15 14:21:10
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
Would you have a link to that specific article?
I support the idea. I am wondering if components could be built with Asphalt. If kept reasonably cold, Asphalt might do. Cold is availible on Mars.
I am also wondering, if you then have need to make glass to construct these things, could you also consider building a fairly conventional multi-paned dome of glass in the sunward directions, and of whatever elsewise.
I would not expect to pressurize the dome, just use it like scales on a reptile, to keep the harshness out of the interior. Keep most of the UV out, keep heat in, keep out the abrasive wind blown dust.
Then put in a low pressure plastic balloon, to conain a further improved environment. A partial pressure suitable to plants, but perhaps not enough for humans. A balloon with a fresh water pond in it. No ice this time open water inside of a balloon inside of a glass dome.
Then the requirments on the balloon would be reduced. Less pressure, temperature lows modified, UV mostly excluded.
Further if it is a pond, it will hold heat at night. Condensation would be collected for use so it would provide that resource. I am also thinking of having a "Boat" in the pond. Either a robot, or a special pressure suit. The pond would be shallow, so that a robot or human arm could reach the bottom and "Cultivate" whatever you are growing.
I did a little research, and tried to make a variety of cattail fit, but I think they might not do good at low pressures, but I am not certain.
http://en.wikipedia.org/wiki/Typha_latifolia
http://www.cattails.info/Types_of_cattail.html
I am not saying don't make your mushroom houses, make them. But if you then know how to make glass, think of this other thing as well. I am thinking that it is just possible that some cold water plant from the Arctic or northern temperate areas of the Earth could be domesticated, and then you would be able to produce a bulk product with a less modified environment.
You did say that things were quiet here, so I thought I would give you some food for thought.
Last edited by Void (2014-09-15 18:51:19)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#62 2014-09-15 15:56:18
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Greenhouse - hydroponics vs soil
Life on Mars?
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#63 2014-09-16 09:18:22
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
Louis,
That is reasonable. A big growth opportunity for any living things requiring elevated soil temperatures, which due to it probably would to grow into large numbers.
But that leaves long periods of time to hibernate.
I have wondered if carbon paths could be existing in the subsoil caused by the passage of electrical currents. If a poor conductance point occurs in an electrical discharge path, then an arc will occur. If an arc occurs it is possible that it would react with atmospheric gasses, such as CO2, and cause a deposit of carbon, which would increase the conductance.
Normal environmental conditions might not be sufficient to promote an electrical discharge sufficient to heat an electrical path in the ground, but I am thinking that during a significant dust storm, there may be a vast energy source to generate static electrical charge, which would be prone to discharge in such a manner through the ground. If this were true, then organisms that profit by being warmed would have tiny havens to periodically experience sufficient temperatures to be active, and to repair damage to their structures, and to multiply. Their food would likely be chemical, but the extra warming would be electrical. Then if an asteroid hit and provided a much larger habitat for a time they would bloom into it, and be able to spread to other locations where electrical habitat may exist.
Last edited by Void (2014-09-16 09:19:50)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#64 2014-09-16 09:29:33
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
GWJohnson,
What I am thinking is multi-layer like skin.
A mesh to be the foundation of the skin structure.
Glass shingles, scales, or feathers if you like to attach to the mesh. They would not form a pressure shell, but would protect the structure from abrasion, UV light, and would tend to hold in heat. Because they would not have to hold pressure, their quality could be much less rugged, however it might be advisable to have a plastic coating on the underside so that if they shatter, they would not shed shards of sharp glass as much. A further advantage is that they would be free to expand and contract with fluctuations in temperature without compressing and pulling at joins that you would have with a standard multipane glaze. They could be much thinner, since they would not hold pressure.
Inside of the Mesh enclosure could be a balloon, partially held unruptured by the mesh. Here again the plastic balloon would be relieved of some tasks, as it would not have to endure UV, it would not have to be the entire method of pressurization (It would have help from the mesh enclosure). It would also be warmer. So your choice of plastics would perhaps be easier to obtain. Also the thickness of the balloon could be reduced.
Should you then have open water, or bags of water in the interior, then you could further buffer the temperature swings.
This is much like a living organism. It's skin is multi-layered, and it has bulk in it's interior to moderate temperature swings.
In this case also nature indicates that a larger size would also help with temperature swings, where a larger volume would have a proportionally smaller amount of "Skin".
Anyway I would try to incorporate this into your mushroom house, or any other type of "Greenhouse".
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#65 2014-09-17 15:08:20
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
According to this website, coefficient of linear thermal expansion for glass plate is 9.0 (10^-6 m/m K). That means a glass pane 2 metres wide, temperature swing from -77°C to -8°C (one day measured by Mars Pathfinder), will result in (77 - 8) * 2 * 9.0 = 1242 * 10^-6 m = 1242 µm = 1.242 mm.
Remember temperature change of 1°C equals 1°K, the only difference is zero is offset.
Calculating for the worst case scenario, the lowest temperature recorded by Viking 2 lander over more than a Martian year is -111°C, and the highest temperature recorded by Mars Global Surveyor at the equator (for the years I was watching published data), was +24°C. Using these numbers, the temperature difference is 135°C, resulting in 2.430 mm expansion. So the window frame requires that much air space between edge of the glass and metal frame. That's the total for both sides of the glass, so roughly half on each side. You could fill the gap with air, or rubber that can compress that much. Glass is held by rubber on both faces (front and back), around all 4 edges. That can be your pressure seal.
Glass will expand vertically as well. For a glass pane 3 metres high, it will sit on the bottom of the window frame, so the top requires 3.645 mm of expansion space above. Again, either air space or rubber that can compress that much.
Gap for thermal expansion is measured in millimetres. You can definitely design window frames that do that.
Offline
Like button can go here
#66 2014-09-17 17:20:55
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
It is good to consider those factors. I have an open mind on the topic, but would be concerned about fatigue of the joins over time in a harsh environment, but of course everything requires maintenance eventually.
I havn't come to it yet, but I am fishing for something that would move in the direction of what you want and yet have the qualities I want. That is I do believe that studying the solutions that nature has employed for organisms to survive harsh situations may be worthwhile to modify the basic concept of holding a volume of pressurized air and allowing sunlight in through windows.
Moving to Mars with Earth based construction concepts is like a fish trying to adapt to land.
Last edited by Void (2014-09-17 17:30:40)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#67 2014-09-17 19:24:56
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
I looked at all the fluoropolymers. I chose PCTFE as the best. Strength, UV durability, transparency, operating temperature range, and gas impermeability.
Living organisms have active maintenance: they constantly replace the outer layer. The cornia of human eyes is modified skin. It's transparent, and no blood vessels. Tears have water and nutrients that cornias require. Tears absorb oxygen directly from air. And tears have an anti-bacterial and anti-mould agents to protect eyes. For further protection, tears are constantly produced behind the eye, wash over the eye, and are directed to tear ducts near your nose. Those tear ducts direct used tears into your nose for disposal. Cornias also are missing several other features of skin: pours, hair, melanin to absorb light including and especially UV.
The lens has something to absorb UV light. A local member who was a retired military photo-tech told me during World War 2 any sailor who had a fresh lens replacement (cataract surgery) was asked to operate signal lights between navy ships at night. Because the lens replacement at that time did not filter UV. At night you use rods in your retna to see. They're far more sensative to light, but do not distinguish between colours. So rods see black-and-white. Cones are smaller and have a colour filter, so one cone only sees one colour. Since rods do not have any filter, they can detect UV as well. Those with lens replacements that do not filter UV, can see UV with their night vision. So navy ships could use UV signal lamps. This same local member also told me that those with lens replacements eventually would grow something over them that blocks UV. So this only worked for a number of months after surgery.
http://www.komar.org/faq/colorado-catar … olor-glow/
If you want an inflatable dome, then you need a polymer that can handle all the capabilies of the first sentence, plus able to withstand sand storms without becoming cloudy. That means natural sand blasting. That's one reason I said glass, instead of inflatable.
There is probably fluorine minerals on Mars, but they're probably just as rare. Another class of polymer that's strong and able to withstand cold are polyimide. I don't know much about them. Kapton is one. It's made of carbon, oxygen, hydrogen, and a little nitrogen. That's all, so ingredients are easy to get. Operating temperature of Kapton HN is −269 to +400°C, so that's great. It has excellent radiation resistance, has been used in space, was part of the Apollo LM. It's less permeable to oxygen than Teflon FEP, but not as impermeable as PCTFE. But the problem: amber in colour. Not completely transparent.
Transmission spectra of various fluoropolymers vs glass:
Transmission of polyimide TF111-615 (I don't know what brand):
Transmission of Kapton: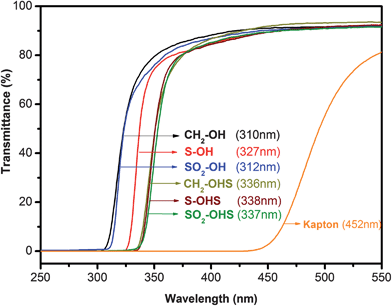
Last edited by RobertDyck (2014-09-18 11:24:50)
Offline
Like button can go here
#68 2014-09-18 10:01:24
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
Very informative.
In the end I would not care how the greenhouses were constructed, just that they were cost effective and functional.
I will argue my side further however, just for kicks and to keep activity alive at this site.
I am sure that structure will be determined in large part by materials available.
Perhaps you have another solution, but I am presuming that your glass panes will be supported by a foundation structure of metal?
In what I have proposed if metal (Or some other material) I might suggest something like the following:
https://www.google.com/images?hl=en&q=e … d=0CD0QsAQ
A balloon structure inside aided by such a corset structure, so that the bulges are small, but many.
Of course that metal mesh will have to be made in such a way that it does not cut the balloon.
While only some of the light will get in, plants will do OK with 1/10 of normal Earth light, but more is better.
The mesh itself would collect thermal energy, and I suppose it would be very nice if it would have some kind of solar cell micro-structure on its outside to collect some electrical energy, but that would be an accessory not necessary.
That structure by itself would be vulnerable to UV, Abrasion, and thermal fluctuations, and so on, so I propose to put tiles, or scales on the outside of some hard material, perhaps glass. On the inner side of the "Glass" could be a UV protectant coating.
Ideally the tiles would be fastened mechanically or magnetically or some of each, and would have the best contour to push in the tiny bulges of the balloon pressure bag held by the corset mesh.
The corset could be formed in complex shapes, and if steel for instance could be welded. Some other materials could be joined into a frame that way as well, if it is not possible to have steel or iron.
None of the components would last forever, but I presume that the corset would be the most durable.
The pressure retaining bag could be patched relatively easy, if small leaks or even tears occurred
The protective tiles, shingles, scales could be replaced with relative ease (I hope), and could be in stock part for replacements. If of a good glass/metal structure, they should be rather durable.
But I have nothing against your more conventional greenhouse plans.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#69 2014-09-18 11:46:56
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
I still argue for fibreglass reinforced PCTFE film for a science mission. For example: Mars Direct. This is a light weight, durable material that can be transported from Earth, and inflated on site. An inflated greenhouse for a science mission wouldn't be all that big, it would grow food primarily as proof that it can be done. It would provide some fresh vegetables, but enough stored food would be brought that astronauts would not starve if the greenhouse failed. To make the film as light as possible, use 2 mil thick PCTFE film, with fibreglass cloth gauze thermally bonded to the inner surface. That provides rip-stop, as well as some reinforcement. The inflated bag would be constrained by straps held down with big tent pegs. This ensures it doesn't blow away in wind, but Mars wind doesn't provide much force. Primarily the straps squish the bag into an oval shape, instead of pure cylinder. And two layers of this film, with a gap between. Pressurize the gap higher than Mars ambient, but less than greenhouse interior. That ensures pressure alone would hold the roof up. Of course that means hold-down straps for both layers. That will require straps for the inner layer to pass through the outer layer, where they will be connected to tent pegs. Where the straps pass through, the strap will be non-woven and non-porous, so it doesn't let air or gas through, and the outer film melted to that section of strap for an air-tight seal. The film would be coated with NASA's spectrally selective coating to control UV and IR. That coating could be applied to the inner surface of the outer film, or outer surface of the inner film. Either way, the coating would be protected from wear. And the gap would be filled with argon gas, for maximum thermal insulation.
The question is what to do with the floor. Mars ground gets very cold at night. Perhaps bubble wrap filled with more argon gas. Under the greenhouse flooring. So hard plastic floor tiles sitting on bubble wrap? The plastic film itself would be a complete bag. Not just a dome sealed to a ring, but a complete bag. That means the plastic film will sit on the ground. That requires preparing the site by using a rake to remove rocks. Once the ground is smooth dirt, then lay the greenhouse on that, and then inflate. One option is to fill the greenhouse floor with Mars dirt, just to weigh down the bag. But again, I would first put bubble wrap on the floor, then dirt on top of that. Then plastic tables holding trays for soil.
For a permanent settlement, construction would be quite different. You still require a pressure envelope without leaks, so that means a floor. You can't just hold it down and expect air won't create a leak through soil. Pressure has a way of finding a way out.
But windows for a greenhouse of a permanent settlement? Here is a decompression chamber used for divers. It holds pressure much greater than anything Mars will endure. It's metal with a transparent window. Not sure what material, but a window in a metal frame. So how to build such a frame is well known.
Last edited by RobertDyck (2014-09-18 14:22:05)
Offline
Like button can go here
#70 2014-09-18 12:59:08
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
Another better example of a glass window in metal frame: Cupola on ISS
Offline
Like button can go here
#71 2014-09-18 14:19:41
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
Having local resources for metal frames, Glass, and plastics will open up lots of options, but what about a start?
I think I would look into the possibility that rather than finished products landed or extraction of minerals, we could consider hard landing basic processed materials, perhaps bags of beeds of materials?
Starting humble, and to achieve a quick result, I would consider bags of plastic hollow beeds which could survive a hard impact. If it were me my first effort would then be to collect and clean those beeds, and using machinery construct a bag for water a bag of water of sufficent size would perhaps fend off the formation of ice overnight. I would perhaps make that bag of a prefered material for that purpose, and put a tarp over it which would serve the purpose of a blanket, and to shield out the UV. Apply a minimum pressurization into it, and perhaps grow a useful plankton in it.
This is not because I don't want greenhouses, but because it would be a fast achievement which might provide some type of food, some type of water recycling/purification for a initial crew of people. Other food provided could be hard landed as well, from Earth, perhaps freeze dried whatever. Then hopefully they would be able to develop a local resource for Glass, Metal or Plastics. Or ideally all three.
Having a food source from simple bags of water, would reduce the size of greenhouse needed, for survival at first, but would not prevent building very big ones eventually. I also suggest that initially very small greenhouses might be built. Actually just large bottles for a batch production. There are perhaps some plants that are rugged that would mostly grow on their own once planted in such terrariums.
Again this is a concept for initial startup, not the eventual better method with greenhouses that people can be in.
Last edited by Void (2014-09-18 14:22:51)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#72 2014-09-18 14:34:27
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
I posted elsewhere my paper for aluminum. And you can harvest hematite concretions for iron, to make steel. Plastics can be made from water, air, and a whole lot of electricity. More sophisticated plastics require other things, like salt. Here are a few pages I wrote, and posted on the local chapter website. Materials and greenhouse pages are from 2001, others from 2002. But they're still relevent.
Offline
Like button can go here
#73 2014-09-18 15:03:03
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
Then there's my idea for a in-vitro chloroplast system. Grow peas to 14 days from germination, harvest leaves, use laboratory equipment that any science mission would have, result is isolated chloroplasts. Put them in a transparent plastic bag of sterile water. Use an aquarium pump to circulate water. For the plastic, use a semi-permeable membrane that lets oxygen through, but not CO2 or large molecules. Water circulation inside the bag will promote oxygen transport across the membrane. And a fan to blow cabin air across the outside. Shine sunlight through a window onto the bag. The window would have the same spectrally selective coating to protect chloroplasts from UV. Take potable water and filter through a reverse osmosis filter to ensure it's sterile, adding water to these bags. Before adding water, filter CO2 recovered from cabin air to ensure it's sterile too, then add that to water under pressure. Let CO2 sit in a canister with water overnight, producing soda water. Add that soda water to the chloroplast bag. Result: chloroplasts convert water and CO2 into O2 and sugar. They also polymerize sugar to create complex carbohydrates. Which carbohydrate depends on which plant you take them from. Peas are the easiest plant from which to harvest chloroplasts. Peas produce pea starch, so you would have pea starch. Have another device draw off some water from the bag and filter with successive filters, keeping starch but returning water to the bag. The result should be a thick starch solution. You could dry that to produce starch powder, or process starch paste for food.
This requires some pea seeds, soil, and a greenhouse. Chloroplasts will last a certain time, then need to be replaced. I got the detailed steps to isolate chloroplasts from a professor at the local university. The catch is if you do this as a normal undergraduate exercise, the chloroplasts last 20 minutes. My assertion is that's due to RuBP. That's an intermediate in the Calvin Benson cycle, also known as the dark reaction. If the O2 to CO2 ratio is too high, then a waste product is made. Plants have a way to recycle that, but one way to reduce that waste is to increase CO2 greatly inside the bag, and the membrane and pump will actively remove O2. This will extend the life of in-vitro chloroplasts, but I want one bag of chloroplasts to last 6 months. That may require something more.
Chloroplasts are made in cells of plants. One chromosome encodes the plasmid of a chloroplast. When a plant cell wants a chloroplast, messenger RNA transcribes the entire chromosome, then instead of being used as a template to make protein, it's transcribed outside the nucleus back into DNA. That strand of DNA is then joined at the ends to make a loop. That's a plasmid. The chloroplast then forms around it. A chloroplasts has 85% of the genes of cyanobacteria. It's basically cyanobacteria that's been enslaved by eukaryotic cells. Chloroplasts rely on two organelles in plant cells to recycle the waste product that I mentioned, they can't do it themselves. But cyanobacteria is self-reliant; actually has 3 pathways for recycling. The pathway used by chloroplasts is most but not all of just one of the cyanobacteria pathways. The missing steps are done by Mitochondria and Peroxisom. What I propose is genetically engineering a pea, adding genes to the chromosome that makes the plasmid for chloroplasts. Add the genes for missing steps of the recycling pathway, and genes for the other two pathways as well. This would still not be enough for chloroplasts to self-replicate, ensuring chloroplasts remain an organelle. However, chloroplasts would be far more efficient. Hopefully this would reduce photorespiration, making peas grow faster. That means greater yield for crops of field peas on Earth. But that's just an excuse to get some company to fund this research. My objective is a plant to be the source of in-vitro chloroplasts for this life support system.
Last edited by RobertDyck (2014-09-18 15:44:43)
Offline
Like button can go here
#74 2014-09-18 15:33:29
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,350
Re: Greenhouse - hydroponics vs soil
Thats good. I just had a problem seeing how to transport enought greenhouse to Mars to keep the "Workers" alive until glass, metals, and plastics manufacturing could be made actual.
A method to supplement imported food with bulk development in the manners we have suggested will make me more of a believer.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#75 2014-09-18 16:14:48
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,394
- Website
Re: Greenhouse - hydroponics vs soil
My page on this: Chloroplast based Life Support
I also looked at how to process starch to make something palatable. I bought a bag of pea starch. Unfortunately the only place I could find was a mill that makes it. Smallest bag was 25kg. I'll have starch for a long time. One recipe: water, starch, bread yeast, and a tiny but of yeast nutrient from a home-brew store. Let the yeast grow at room temperature for 3 days. Then cook in a microwave oven. The result has the smell and aroma of freshly baked bread. Texture is like pudding, colour is translucent white. If you don't add yeast, it's completely tasteless. Yeast not only adds flavour, it adds protein, lipids, and the complete vitamin B complex. Everything but B12. There is a yeast-like organism you could add that produces B12, but I just used traditional bread yeast. Not a lot of protein, but enough to substitute potatoes. Yeast nutrient is diammonium phosphate; it adds nitrogen and phosphorus in a form that yeast can metabolise. Need nitrogen for protein, and both nitrogen and phosphorus for DNA.
Recipe
1/2 cup (125ml) pea starch
2 cups (500ml) warm water
1/8 teaspoon traditional bread yeast
1/4 teaspoon yeast nutrient (from a wine making store)
Take one ladle full into a soap bowl.
Cook in microwave at full power for 30 seconds. Stir.
Cook at full power another 30 seconds.
Should be mostly gelled with some liquid on the sides.
Stir thoroughly. Will form a translucent gel with consistency of pudding.
Offline
Like button can go here