New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2011-12-16 17:38:57
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,141
- Website
Re: Mobile Energy Storage in a Mars Colony
Don't worry about imperial units for me, I speak both. I just think a bit better in imperial, because that's what we used the most when I worked in the defense industry long ago. It's just practice, not a problem understanding.
Hadn't thought about methanol, might be a good idea. Not so sure about methanol-in-contact-with-N2O4 or any other oxidizer. Pre-mixed flammables and explosives are a bit scary. A lot of folks don't realize that methanol is a skin-absorption nerve poison. It is possible to absorb a lethal dose through the skin of your hands, if you keep them immersed for a few hours in a day. But, it's a very nice fuel material, solvent, etc, if you just treat it with a tad of respect. It is a bit corrosive to a lot of materials, especially polymers. Steel works OK, stainless is better. Aluminum, not so much.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#27 2011-12-16 18:02:33
Re: Mobile Energy Storage in a Mars Colony
Some method of storing Methane will be necessary, true, but perhaps not quite as much as you suggest, in that with the exception of rocketry there will be little requirement for long term storage of methane. Methanol and other storeable chemical energy storage media will function in most other applications. Before the colony really gets into rocketry (which will require cryogenics anyway) most of the methane that will be produced will simply be used immediately after for chemical synthesis of other things.
With regard to pressure vessels, the colony is without doubt going to have need of the creation of pressure vessels of all sorts. Any inhabitable space, properly considered, will be a pressure vessel. For chemical synthesis of tens if not hundreds of vital chemicals, pressure vessels will be required at some stage. The concern, as usual, is cost as opposed to ability. In this case, we might look at pressurized methane or even well-insulated cryogenic methane.
-Josh
Offline
Like button can go here
#28 2011-12-17 11:47:41
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,141
- Website
Re: Mobile Energy Storage in a Mars Colony
I'm thinking some sort of steel-making plant is one of the first things a permanent base will need. Shipping the plant once (whatever it really is) is cheaper over the life of the base (decades+) than shipping steel stocks from Earth, almost no matter what the cost to LEO is.
The other is some sort of plastics-making plant. Not everything should be made of steel. Aluminum can come later. If you've got plastics and steel, you can pretty well cope for a while.
On Mars, concrete is going to be a bit of a problem, as limestone does not seem to be available, although water is. There has to be some equivalent with the minerals widely available there. It may take a while experimenting before we find it. But find it we must, concrete is just too useful to do without it. An ice-regolith composite reinforced by steel bars might serve in some applications, as long as material temperatures do not exceed 0 C.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#29 2011-12-17 14:46:22
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mobile Energy Storage in a Mars Colony
I'm thinking some sort of steel-making plant is one of the first things a permanent base will need. Shipping the plant once (whatever it really is) is cheaper over the life of the base (decades+) than shipping steel stocks from Earth, almost no matter what the cost to LEO is.
The other is some sort of plastics-making plant. Not everything should be made of steel. Aluminum can come later. If you've got plastics and steel, you can pretty well cope for a while.
On Mars, concrete is going to be a bit of a problem, as limestone does not seem to be available, although water is. There has to be some equivalent with the minerals widely available there. It may take a while experimenting before we find it. But find it we must, concrete is just too useful to do without it. An ice-regolith composite reinforced by steel bars might serve in some applications, as long as material temperatures do not exceed 0 C.
GW
I agree we would want to have a scaled down steel plant at an early stage - maybe even Mission 1.
Plastics are problematic I believe. People who have worked with polymers before say it is a very complex process that might be beyond the abilities of a tiny colony. We will have glass, glass fibre, reconstitued basalt, metals and bamboo (wonderfully fast growing and versatile bamboo) for tools, containers, vessels and so on.
Limestone is an issue (for steel making as well I think). The evidence on calcium carbonate being present on Mars is a bit ambiguous. It does appear to be there - somewhere!
I think in the early years Mars brick with regolith cover will work for most construction requirements. I like the idea of Roman arch over trenches dug by mini diggers (permafrost being melted with microwave pulser).
Interesting what you say about ice-regolith composite. I have wondered about using ice for airlock doors: the ice is melted to "unlock" the door and water is frozen to create the lock. We certainly need some creative thinking along these lines.
Ice-regolith slabs might offer some additional protection against cosmic radiation - so the colonists can work out in the open under those protective layers.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#30 2011-12-17 16:24:34
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,141
- Website
Re: Mobile Energy Storage in a Mars Colony
Cosmic radiation is not the bugaboo that everyone thinks. The max radiation exposure occurs during solar minimum, and is just a tad beyond the yearly dose we now allow astronauts to receive anyway. At solar maximum, this exposure is cut in half by the solar wind, so that for most of the 22-year sunspot cycle, cosmic ray doses are under what is already allowed.
The real radiation danger is not cosmic rays, it is solar coronal mass ejections. Those, not cosmic rays, are what our magnetic field shields us against. Mars has none. Fortunately, these are brief events, a few hours to a day or so. About a meter of water or dirt works pretty good as a shield. Nothing special there.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#31 2011-12-17 16:57:36
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mobile Energy Storage in a Mars Colony
Cosmic radiation is not the bugaboo that everyone thinks. The max radiation exposure occurs during solar minimum, and is just a tad beyond the yearly dose we now allow astronauts to receive anyway. At solar maximum, this exposure is cut in half by the solar wind, so that for most of the 22-year sunspot cycle, cosmic ray doses are under what is already allowed.
The real radiation danger is not cosmic rays, it is solar coronal mass ejections. Those, not cosmic rays, are what our magnetic field shields us against. Mars has none. Fortunately, these are brief events, a few hours to a day or so. About a meter of water or dirt works pretty good as a shield. Nothing special there.
GW
Thanks for the clarification GW. While we are on the subject, would you know if water irradiated by a coronal mass ejection is drinkable afterwards - or should I say potable i.e.non-injurious to health.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#32 2011-12-17 20:52:40
Re: Mobile Energy Storage in a Mars Colony
GW- I'm not really so sure about that, on radiation, actually. Firstly, take a look at this section of the wikipedia article on MaRIE, which was an instrument used to measure the radiation environment around Mars. The following image (from this site) shows that we can expect at least about 14 rem per year on the surface of Mars.

According to this, NASA limits astronauts to 25 rem per space shuttle mission. In that case, I retract that statement; however, even if it is "only" 14 rem per year, that will certainly build up over the course of a lifetime.
That said, given that the colony's structures are going to have to be covered in about 4+ meters of regolith anyway (that is, IMO, the simplest and safest way to deal with the high internal pressures), radiation within the habs will be reduced to very low levels and so long as the person is not outside should not be an issue.
-Josh
Offline
Like button can go here
#33 2011-12-17 21:41:25
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mobile Energy Storage in a Mars Colony
GW- I'm not really so sure about that, on radiation, actually. Firstly, take a look at this section of the wikipedia article on MaRIE, which was an instrument used to measure the radiation environment around Mars. The following image (from this site) shows that we can expect at least about 14 rem per year on the surface of Mars.
http://www.solarstorms.org/MarsDosages_ … rie_br.jpg
According to this, NASA limits astronauts to 25 rem per space shuttle mission. In that case, I retract that statement; however, even if it is "only" 14 rem per year, that will certainly build up over the course of a lifetime.
That said, given that the colony's structures are going to have to be covered in about 4+ meters of regolith anyway (that is, IMO, the simplest and safest way to deal with the high internal pressures), radiation within the habs will be reduced to very low levels and so long as the person is not outside should not be an issue.
Certainly for the first mission or two, I don't think people are going to be outside that much. If the mission was structured the way I envisage I guess there would be a week during which there was a lot of external work as supplies were gathered together from the various robot drops. A lot of "outside" time would probably be spent in a pressurised rover/digger.
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#34 2011-12-18 10:00:40
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,141
- Website
Re: Mobile Energy Storage in a Mars Colony
For annual cosmic radiation exposure limits used for NASA astronauts, see Table 1 from http://srag.jsc.nasa.gov/Publications/T … chmemo.htm for the 50 rem that I have been using. You can glean from other sites that cosmic radiation fluxes in near-Earth space are modulated by the strength of the solar wind to between 60 rem and around 30 rem.
Most of the 22-year solar cycle, in-space exposures are within the 50 rem limit. On the surface, remember that exposure is cut in half, because the planet beneath your feet is a shield against half the sky. Shielding might not be true for small bodies like asteroids.
The problem is Table 2, the career exposure limits to cosmic radiation, which limit you to around 2 or 3 years in space. Remember, there really isn't a practical shielding technique for radiation this energetic, because of the secondary showers it creates.
For an exploration mission to Mars, a 2 year voyage is OK, but don't ask them to fly again, under these rules. With thick roofs or underground habitations on the moon and Mars, exposure should be tolerable, but will likely violate career limits after 5 years or so, due to secondary shower effects. The only real thing a meter or 10 of regolith can protect you from is solar flare radiation, not cosmic rays.
Shoot, we get hit with cosmic rays right here on Earth, some primary, some secondary shower coming down from our own atmosphere, the mass of which is the real shield. Our magnetic field turns solar flare particles, not cosmic rays.
BTW, average Earth natural background radiation (of all types) is around 0.3 rem annually, the top third of which is radioactive emissions from coal plants. This value varies widely around the planet by a factor of 10 or more. It's pretty variable.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#35 2011-12-18 17:19:52
Re: Mobile Energy Storage in a Mars Colony
GW- At some point, piling on enough regolith will block out a pretty significant proportion of the radiation anyway. For comparison, the Earth's atmosphere is the same in terms of mass overhead to 4 m of regolith, though the Earth obviously does have a magnetic field as well, no matter how weak. I think that if we could get radiation down to about 1 rem/year that would be plenty good.
-Josh
Offline
Like button can go here
#36 2011-12-18 19:24:43
- louis
- Member
- From: UK
- Registered: 2008-03-24
- Posts: 7,208
Re: Mobile Energy Storage in a Mars Colony
This reference seems to support Josh's view:
Let's Go to Mars...Google on: Fast Track to Mars blogspot.com
Offline
Like button can go here
#37 2011-12-19 09:29:31
- GW Johnson
- Member
- From: McGregor, Texas USA
- Registered: 2011-12-04
- Posts: 6,141
- Website
Re: Mobile Energy Storage in a Mars Colony
Then again, you have to consider where the exposure standards came from: best guesses based on folks exposed. Linear dose rate models do not work. The low-exposure limits are very, very crude best-guesses based on aging Japanese A-bomb survivors, and troops exposed in tests in Nevada in the 50's.
Most old guys like me were exposed to a lot more radiation than 1 rem a year just from watching early-model TV's with unshielded Klystron tubes in them. Momma always said don't sit too close, but never knew why. Now we know: a lifetime's X-ray in a week to a month, sitting within 6 feet. I'm still here. Most of us this age still are (for a little while yet).
So, I don't really see much problem with 25-50 rem /year exposure "for a while". The career limits are more doubtful. I honestly don't know. The original WW2 standards were 25 rem a year, no career limit. A lot of those guys had problems, a lot didn't. Hard to know why and how.
I guess my point is that the low-dose standards are really guesses, not such hard science after all. The harder science is the high-dose standards. Things like 25-50 rem in a week, that's going to kill some percentage of those exposed, and within days. It's a certain thing. We've seen it and measured it, directly.
GW
GW Johnson
McGregor, Texas
"There is nothing as expensive as a dead crew, especially one dead from a bad management decision"
Offline
Like button can go here
#38 2018-11-25 17:28:20
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,453
Re: Mobile Energy Storage in a Mars Colony
This is not a problem in the early stages as its ok to start small but could be the reason for moving if we can not sustain life where we land.
I also now realize that I forget more than I knew when reviewing the content of research for a topic....
Offline
Like button can go here
#39 2018-12-04 18:43:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,453
Re: Mobile Energy Storage in a Mars Colony
The first problem faced by man is coming up with a working fluid from Mars atmospheric pressure on the Martian surface when it only averages 600 pascals (0.087 psi; 6.0 mbar), about 0.6% of Earth's mean sea level pressure of 101.3 kilopascals (14.69 psi; 1.013 bar).
Once we have the desire volume of pressure desired for any of the gaseous content that mars has; then we can build a system for processing it and of its energy required to do so.
Which leads to what can we do with solar as in concentrating and those working fluids in closed or open designs. The Stirling engine comes to mind.
https://www.grc.nasa.gov/www/tmsb/dynam … solar.html
digitalassets.lib.berkeley.edu/techreports/ucb/text/EECS-2007-172.pdf
https://www.springer.com/cda/content/do … 823-c2.pdf?
www.iieta.org/sites/default/files/Journals/IJHT/35.03_06.pdf
https://en.wikipedia.org/wiki/Solar_thermal_energy
We go through so many topics and here is a related to the atmosphere use.
Mars Atmospheric Kinetic Engine
http://www.travisdeyle.com/files/public … mPaper.pdf
Inflatable Membrane Solar Concentration Systems for Space-Based Applications
Offline
Like button can go here
#40 2018-12-06 19:25:47
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,453
Re: Mobile Energy Storage in a Mars Colony
I got thinking about how to concentrate the intake of the co2 inlet for the compressor to be able to move it to a storage tank but under higher pressures. The through put was to be created on a circular track or road for a solar power rover to just drive at velocity with a funnel shaped opening to use as a ram intake to boost the incoming pressure to allow for a compressor to work more easily.
https://en.wikipedia.org/wiki/Velocity_stack
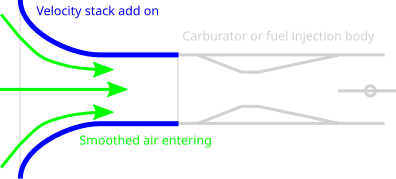
Basically using a rocket engine nozzle in reverse to create a greater pressure via motion.
Storing on the vehicle to be released to a much larger tank when its its stopped.
Offline
Like button can go here
#41 2018-12-08 08:39:33
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Mobile Energy Storage in a Mars Colony
There are devices known as ejectors. They consist of a jet of gas that provides the motive power for the gas that is to be drawn in, a mixing chamber and then a nozzle and diffuser. They are widely used in vacuum pump inlets to get a very low pressure on the upstream end without having a very large machine, which is exactly what we will need on Mars. The motive gas is diverted from the vacuum pump outlet stream and recycles back to the pump. Alternative arrangements use steam or another gas, or a liquid such as water, for the motive medium.
Offline
Like button can go here
#42 2018-12-08 09:38:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,453
Re: Mobile Energy Storage in a Mars Colony
The injector works as a result of greater pressure on the one side to allow flow through the device. Since its only flows in one directionis sort of like a check valve as well but electronicically controlled.
So we might be able to combine vehicle motion as the ability to compress mars Co2 with the help of injectors that allow the flow of pressure into a tank for storage as a function of collection.
Offline
Like button can go here
#43 2018-12-08 15:12:16
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,069
Re: Mobile Energy Storage in a Mars Colony
Caveat ... I am not an engineer. I am pretty sure there are members of the forum with significant engineering experience. I am hoping this question may be of interest:
Picking up on SpaceNut's image of a gas compression method, I am reminded of ram jets, which have the advantage of simplicity when compared to turbojet engines in particular. I am wondering if there are useful trades which might make a rotating arm with ramjet tips worth considering for collecting Martian atmosphere for input to a compressor. While SpaceNut has considered a moving vehicle as a location for ramjet style gas compression, I am wondering if a fixed installation might be worth trying, with a combination of technologies to arrive at an optimum efficiency?
To boil it down, I ** think ** the question could be reduced to: is a fan better than a ram for pre-compression of Martian atmosphere?
The injector works as a result of greater pressure on the one side to allow flow through the device. Since its only flows in one directionis sort of like a check valve as well but electronicically controlled.
So we might be able to combine vehicle motion as the ability to compress mars Co2 with the help of injectors that allow the flow of pressure into a tank for storage as a function of collection.
(th)
Offline
Like button can go here
#44 2018-12-09 05:10:16
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Mobile Energy Storage in a Mars Colony
I did consider a tip jet arrangement for a possible Mars helicopter. I couldn't make an enormous rotor produce sufficient lift for expeditionary purposes with a tip speed of M=0.85. The very low density of the atmosphere was the main problem here. Nonetheless, putting a diffusion device on each end of a long, centre pivoted arm rotating at high speed would be possible. All the structural problems for this have been solved. However you would use a lot of the pressure you generate just getting the product to flow back up the arm to the centre where it can be tapped off and quite a bit of power overcoming the windage of the arm.
Offline
Like button can go here
#45 2018-12-09 07:33:27
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,069
Re: Mobile Energy Storage in a Mars Colony
For elderflower,
Thank you for giving the question a boost! Your point about the drag on a single arm inspired me to wonder about a continuous structure. If this link works, it should show a view of the nautilus from nature, and a human design for an impeller.
https://www.mnn.com/leaderboard/stories … ficiencies
For Mars, such an impeller would (probably) be made as light as possible within the constraint of requirements for strength, since (I'm assuming) it would need to spin at a fairly high rate to deliver useful volumes of atmosphere to the next stage of a compression system.
My guess (at this point) is that the best place for a filter system would be outside the spinning device, because that would be a convenient location for maintenance.
(th)
I did consider a tip jet arrangement for a possible Mars helicopter. I couldn't make an enormous rotor produce sufficient lift for expeditionary purposes with a tip speed of M=0.85. The very low density of the atmosphere was the main problem here. Nonetheless, putting a diffusion device on each end of a long, centre pivoted arm rotating at high speed would be possible. All the structural problems for this have been solved. However you would use a lot of the pressure you generate just getting the product to flow back up the arm to the centre where it can be tapped off and quite a bit of power overcoming the windage of the arm.
Offline
Like button can go here
#46 2018-12-09 16:54:07
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,453
Re: Mobile Energy Storage in a Mars Colony
The atmospheric pressure on the Martian surface averages 600 pascals (0.087 psi; 6.0 mbar), about 0.6% of Earth's mean sea level pressure of 101.3 kilopascals (14.69 psi; 1.013 bar).
This at a minimum is 169 times for a mars to earth transision and we need some pressures to be even higher for some of the processes to work.
So why try to go for all of the difference when multi stage would get the same result as we can make use of the natural mars rythm for making compression happen via solar and nightly cooling.
So where is NASA with this? A NASA-supported scientist is learning how to use carbon dioxide--the main gas in Mars' atmosphere--to harvest rocket fuel and water from the red planet.
Offline
Like button can go here
#47 2018-12-09 20:48:02
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,499
Re: Mobile Energy Storage in a Mars Colony
SpaceNut,
This still looks like a job for RamGen's purpose-built supersonic inlet CO2 compressor. Even though DoE funds this project to suck in CO2 from coal-fired power plants here on Earth, it would still work on Mars. The problem with blades is the massive wave drag on the blades near and above Mach 1. Mach 1 is much lower in the thin Martian atmosphere. RamGen was demonstrated at up to Mach 3. The machining of the blisk (that's a blade-disk) is simpler, even if the fluid dynamics involved requires a supercomputer to optimize the ramp geometry to use the shockwave to assist with compression of the CO2. The primary advantages are cost of machining (it's basically a dog bowl with special ramps machined into the edges), comparatively tiny size (10-to-1 to 12-to-1 compression per stage) and thus overall machine mass, and the ability to recuperate a substantial amount of input mechanical work using thermal energy in the compressed CO2.
Offline
Like button can go here
#48 2018-12-09 21:04:11
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Mobile Energy Storage in a Mars Colony
About an energy storage chemical compound, how about ethylene glycol, glycolaldehyde, glyoxal, glycolic acid, glyoxylic acid and oxalic acid? They are essentially different reduction states from hydrogen reduction of two molecules of carbon dioxide. Would they store both chemical energy as reduced form of carbon dioxide and kinetic energy as condensed forms? Those compounds and their esterification products are mostly liquids on Earth so they are likely liquids or solid on Mars.
Last edited by knightdepaix (2018-12-09 21:04:49)
Offline
Like button can go here
#49 2018-12-09 21:23:30
- knightdepaix
- Member
- Registered: 2014-07-07
- Posts: 239
Re: Mobile Energy Storage in a Mars Colony
Some method of storing Methane will be necessary, true
MARS DATA:
Natural temperature of space craft at Mars Orbit: 5° C
Natural temperature of space craft at Earth Orbit: -47° C
(These values can be raised or lowered by the surface texture and color.)Note: Liquid Nitrogen boils at -196°C (77K) at 1 atm pressure. Critical temperature is -146.9°C (147K).
Insolation at top of atmosphere:
- Semi-major axis...........................560 W/m^2 (43% that of Earth)
- Aphelion...................................... 468 W/m^2
- Perihelion.....................................681 W/m^2(Since Mars spends more than 1/2 of its time further from the sun than the semi-major axis, Robert Zubrin suggests an average value of 500 W/m^2 which is easy to remember.)
On the surface, the thin dusty atmosphere reduces the above values by about 100 to 200 W/^2.
Average Temperature: -63° C or 215K
Highest Equatorial Temperature: 20° C or 293K
Coldest (South) Polar Temperature: -132° C or 141 KThe Blackbody temperature for Mars is: -67° C or 211K
(For Comparison, the Blackbody temperature of: Venus 11° C or 283 K. Earth -23° C or 249K. Ceres is -136° C or 137K. Callisto is -178° C or 95K.)References:
"Moons and Planets" by W.K. Hartmann
"Entering Space" by Robert Zubrin
Wikipedia
"Mars: A Warmer Wetter Planet" by J. S. Kargel
"Terraforming" by M. Fogg
How about methyl formate from essentially reacting 1 part of methane and 1 part of carbon dioxide? Methyl formate has 173K melting point and 305K boiling point in Earth atmosphere. In Mars, it would melts and boils at lower temperature. As a result, methyl formate could then be in most cases a liquid on Mars, a solid in the coldest situations and a gas in the hottest situations. Three phrases would be possible; is it helpful for energy storage? According to Wikipedia page, methyl formate is also an insecticide.
Last edited by knightdepaix (2018-12-10 20:03:04)
Offline
Like button can go here
#50 2018-12-10 14:03:07
Re: Mobile Energy Storage in a Mars Colony
Methyl formate is an option, sure. If we're going that route though I would think we might go for Methanol. Methanol is produced by reacting H2 with CO over a zinc catalyst at elevated temperature and pressure. It freezes at 175 K and boils at 338 K (under one atmosphere). Of course you still need an oxidizer, which is the real problem. LOX boils around 90 K, way below Martian ambient. You'd probably want something more like the kind of oxidizer used in solid rockets: A peroxide, perchlorate, or nitrate. The problem is that all of these are complicated to make, corrosive, and use up a lot of energy. You could store Oxygen as a compressed gas. At Martian temperatures and 100 atm, the density would be around 175 kg/m^3 according to the ideal gas law, which isn't awful. Worth mentioning that Oxygen will be a supercritical fluid at this temperature/pressure so the ideal gas law is likely to be way off. High-pressure gas tanks are heavy and expensive too.
-Josh
Offline
Like button can go here