New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2018-01-12 14:36:41
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Sodium-Iron battery?
I've been thinking about potential battery chemistries that use the most abundant elements on Earth, since nickel, lead, and lithium are not abundant enough for us to replace all our power usage as a species with intermittent renewables. Sodium-ion is promising, and I've been thinking about potential replacements for nickel in nickel-iron batteries, which led to the idea for a sodium-ion battery that would use sodium hydroxide as an electrolyte. In particular, a simple design made from easily accessed materials that could be added to the open source ecology global village construction set.
I put the idea up on Halfbakery, and so far no-one has given an explanation of why it wouldn't work. I can't see a reason it wouldn't myself (hence why I'm sharing it), but perhaps someone here is more knowledgeable about electro-chemistry than I am.
The battery comprises an iron plate serving as an anode, a (probably tin coated) graphite [okay, should be cellulose] cathode, and sodium hydroxide as an electrolyte. During charging, sodium ions would migrate to the cathode and become intercalated into it, whilst hydroxyl ions would react with the iron to form iron hydroxide. When the battery is discharging, this would occur in reverse.
The advantage this battery has is that the materials needed to produce it are all incredibly cheap. The disadvantage is that it may not work. I really have no idea. Low capacity isn't an issue if the cost is low enough, since they would only be used for stationary applications anyway.
Use what is abundant and build to last
Offline
Like button can go here
#2 2018-01-12 16:29:30
- Midoshi
- Member
- From: Colorado
- Registered: 2007-07-14
- Posts: 157
Re: Sodium-Iron battery?
Terraformer, maybe I am mistaken, but what you describe sounds similar to a nickel-iron battery, though they use a nickel cathode instead of carbon (obviously), and usually employ a potassium hydroxide solution (though it appears sodium hydroxide can also be used).
https://en.wikipedia.org/wiki/Nickel%E2 … on_battery
It seems some researchers have recently worked on updating this old technology by adding carbon to the mix:
https://news.stanford.edu/news/2012/jun … 62612.html
So it seems that you have hit upon a good idea, and the details are in the engineering.
"Everything should be made as simple as possible, but no simpler." - Albert Einstein
Offline
Like button can go here
#3 2018-01-13 04:27:53
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
It's similar to an NiFe battery at the iron end, with the same redox reaction. At the other end, it would remove sodium ions from the solution to provide the hydroxyl ions for the reaction, rather than have nickel ion give them up.
Use what is abundant and build to last
Offline
Like button can go here
#4 2018-01-13 12:30:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,917
Re: Sodium-Iron battery?
https://en.wikipedia.org/wiki/Nickel%E2 … on_battery
The nickel-iron battery (NiFe battery) or "edison cell" is a storage battery having a nickel oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide (lye can be used as a substitute). The nickel–iron battery (NiFe battery) is a rechargeable battery having nickel(III) oxide-hydroxide positive plates and iron negative plates, with an electrolyte of potassium hydroxide. The active materials are held in nickel-plated steel tubes or perforated pockets. The Nickel-Iron batteries, are idea for solar power and renewable energy products, Iron Edison is providing people with off-grid, clean energy solutions.
Offline
Like button can go here
#5 2018-01-17 13:59:33
Re: Sodium-Iron battery?
NiFe and NaFe batteries have the advantage of being quite similar. They only differ by one letter!
I like the idea but I don't know enough about battery tech to confidently say if it will work or not.
-Josh
Offline
Like button can go here
#6 2018-01-18 05:08:22
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
Tin coated cellulose has been used as an anode in sodium-ion batteries - Kurzweilai. Since lye soaked cellulose should conduct electricity (it works with salt water, at least), I wonder if we could dispense with the tin coating, allowing us to build a battery out of iron, paper, and lye?
I really ought to dig out my chemistry kit in that case. It shouldn't be difficult to test.
Use what is abundant and build to last
Offline
Like button can go here
#7 2018-01-18 16:44:56
Re: Sodium-Iron battery?
I think it's problematic to build a sodium battery that uses water for any part of it as metallic sodium and water tend to react rather violently.
I suspect you'll end up with something more like an electrolysis cell, where reduction of the Iron anode reduces the required voltage for electrolysis somewhat.
In that case NaCl might actually be an even better electrolyte as FeCl2 and FeCl3 are soluble in water while Fe(OH)2 and Fe(OH)3 are less so. But I digress.
This would work as a molten salt battery, presumably, but that's a bit harder than Iron, paper, and lye. It looks like Lithium Ion Batteries use LiXFy compounds (For example LiPF4) in various organics (Wikipedia suggests ethylene carbonate, dimethyl carbonate, and diethyl carbonate).
Also, I learned today that in a lithium ion battery Lithium is being reduced, and Cobalt is being oxidized from the +4 to +3 state. Interesting stuff!
It looks like you might be able to get away with Methanol, actually. NaCl is somewhat soluble and FeCl3 is very soluble. Wiki doesn't say anything about FeCl2, though, so you may want to check. I don't remember offhand how to check that it won't produce chlorine gas, but you'll definitely want to look into that as well.
I take it back--Sodium reacts violently with alcohols. My bad!
The trick I suppose is finding a solvent that won't react with Sodium but will dissolve a compound of Sodium and Iron. Formamide might do the trick but information is hard to find and that's not a nice chemical to deal with because it has the unfortunate habit of sometimes decomposing to HCN gas.
-Josh
Offline
Like button can go here
#8 2018-01-19 06:44:29
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
I'm not sure the batteries operation would actually involve metallic sodium. It depends on how the sodium is trapped in the anode. Is it kept in metallic form, or as positive ions in a negatively charged structure? If the latter, there shouldn't be a problem.
The impression I get from the KurzweilAI article is that the cellulose anodes store them as ions, so it should be okay.
Last edited by Terraformer (2018-01-19 06:45:47)
Use what is abundant and build to last
Offline
Like button can go here
#9 2018-01-19 07:07:24
Re: Sodium-Iron battery?
The thing is that batteries work based on a Redox reaction. So before charging you have Na+ and Fe0. Then you charge it by taking electrons from Fe and giving them to Na to make it neutral. When you're discharging you do the opposite. When the battery is 100% discharged there's no metallic sodium but by necessity there is when it's got energy to give up.
The battery reaction (assuming we use chlorides) is as follows:
Fe+3 NaCl+energy <-> FeCl3 + 3 Na
The flow of electrons from Fe to Na is the current flowing through the wires attached to the battery.
-Josh
Offline
Like button can go here
#10 2018-01-19 08:26:55
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
Again, that doesn't seem to be how it works in the cellulose anode. It seems to be instead storing electrons as a negatively charged structure, with the charge balanced by the inclusion of sodium ions.
As I understand it, in lithium-ion batteries, the lithium starts in a neutral state when it's discharged. and is stored as lithium ions when the battery is charged. But either way, it gets stored as ions at some point.
How I'm thinking the battery would work is, electrons are put into the cellulose anode, which exchanges hydroxyl ions for sodium ions to balance the charge, resulting in a negatively charge cellulose mesh incorporating positive sodium ions to keep the charge neutral. The hydroxyl ions are then stored by reacting with the iron cathode to form iron hydroxide, releasing electrons which completes the circuit. This is reversed when the battery is discharged.
Use what is abundant and build to last
Offline
Like button can go here
#11 2018-01-19 16:51:27
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
Come to think of it, I wonder if something that forms an alloy with sodium could be used as the anode? The other metal could possibly protect the sodium from contact with water in its metallic state. Or maybe something that forms a compound with it that can't be dissolved by water.
As far as I can tell, the proposal succeeds or fails on the question of isolating metallic sodium from water. If it's stored as ions, no problem. If it's stored away from the water, also no problem.
Use what is abundant and build to last
Offline
Like button can go here
#12 2018-01-19 17:08:34
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: Sodium-Iron battery?
A mercury electrode is used in the electrolysis of salt. The sodium liberated from the salt is absorbed by the mercury, forming a liquid amalgam. The mercury amalgam is then extracted from the cell and is reacted with water to produce sodium hydroxide (caustic soda), liberating hydrogen. The mercury is recycled back to the electrolytic cell. This is a well established industrial process. I never heard of its having been tried as the basis of a battery, though.
Offline
Like button can go here
#13 2018-01-19 19:18:59
Re: Sodium-Iron battery?
Again, that doesn't seem to be how it works in the cellulose anode. It seems to be instead storing electrons as a negatively charged structure, with the charge balanced by the inclusion of sodium ions.
Electrochemistry has never been my strength, but I don't think that's what happens in a battery. I read the article n KurzweilAI and the very similar article on the UMD news site and I'm sorta unclear on various points.
The novel development here seems to be that they used wood coated with tin instead of a different substrate, and that this improved the lifetime of the battery by deforming rather than snapping as the coating changed shape.
I don't believe that batteries "store" electrons; If they did, that would make them capacitors. Batteries are fundamentally chemical things. Chemical reactions happen at the electrodes that absorb or release electrons. Fundamentally, in order to work, reduction or oxidation needs to be happening at each electrode. Specifically, reduction happens at the cathode and oxidation happens at the anode.
For either of those to happen, the oxidation state of something has to change. Otherwise, it's not a battery. Most batteries, it seems, use the difference in electron affinity of two different metals to create an emf between their two terminals.
The battery being proposed is exactly that: Iron has a higher electron affinity than Sodium. According to Wikipedia's table of Standard Electrode Potentials, the reaction:
Na+ + e- -> Na(s)
Has a standard electrode potential of -2.71 V, and:
Fe3+ + 3e- ->Fe(s)
Has a standard electrode potential of -0.04 V.
Putting the two together you'd expect a cell voltage of 2.67 V (minus inefficiencies). But the important thing is that these reactions are actually occurring at the electrodes, with the movement of negatively charged ions (such as Cl-) completing the circuit. (If you were to do Fe3+ + e- -> Fe2+ instead of going all the way to the metal, you could actually get a cell voltage of 3.48 V instead! In a real cell you would expect voltage to be 3.48V until the Fe3+ is used up, and then 2.27 V thereafter).
By the way, this is basically what happens in Lithium Ion batteries. At the anode LiC6 -> Li+ + C6 + e-, and at the cathode CoO2 + Li+ -> LiCoO2. The fact that Co doesn't go anywhere hides it, but the Cobalt is actually changing oxidation states from +4 to +3 and absorbing an electron.
There's a few ways to try to make this kind of cell work. The traditional way would be to find some nasty organic chemical in which Iron and Sodium compounds are both soluble and run with it. Another way (for stationary applications) could be a kind of gravity cell. You might have water and another liquid, totally insoluble in each other. The liquids both dissolve the relevant compounds of Fe and Na. Cathode (where Fe metal is produced) is in water and the anode (where Sodium oxidizes) is in some organic compound that dissolves salts but doesn't react with sodium.
It's a tough problem because Sodium is so reactive, but it should be possible. Lithium batteries use slightly-polar organics.
-Josh
Offline
Like button can go here
#14 2018-01-21 03:40:00
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
I'm trying to find out how the lithium is stored in the anode of current batteries. Everything I'm reading is pointing towards them being stored in ionic bonds, rather than the ions being reduced to lithium metal. Which fits with what I know about electrons in graphite. If the sodium atom doesn't have an electron to donate, it won't react with water.
To maintain the charge balance in the cathode, an equal number of some of the positively charged intercalated lithium ions are dissolved into the electrolyte solution. These travel over to the anode, where they are intercalated within the graphite. This intercalation reaction also deposits electrons into the graphite anode, to ‘tie’ up the lithium ion.
During discharge, the lithium ions are de-intercalated from the anode and travel back through the electrolyte to the cathode. This also releases the electrons that were tying them to the anode, and these flow through an external wire, providing the electric current that we used to do work
That doesn't sound like lithium being stored as metal. That sounds like a negatively charged lattice storing positively charged ions.
Use what is abundant and build to last
Offline
Like button can go here
#15 2018-01-21 10:54:06
Re: Sodium-Iron battery?
Hmmmm....
Based on some research it seems like you're right that Lithium does exist in the +1 state in LiC6. That would mean that the graphite layers between which the Li is intercalated have a charge of -1 per 6 carbon atoms.
However, I think it would be going too far to say that because the Li exists in the +1 state that this compound is unreactive. Looking at Wiki's table of standard electrode potentials, we see:
Li+ + e- -> Li (s)
Has a standard electrode potential of -3.04 V and:
Li+ + C6(s) + e- -> LiC6(s)
Has a standard electrode potential of -2.84 V. For comparison Na neutralization is -2.71 V.
I guess what I'm saying is that it might well react with water to form LiOH and H2. For example,
Li3N + 3 H2O -> 3 LiOH + NH3
Will happen spontaneously even though Lithium Nitride is otherwise stable.
I guess this does change my understand of lithium ion batteries though. It's actually powered by Carbon and Cobalt--Lithium is just an intermediary.
-Josh
Offline
Like button can go here
#16 2018-01-31 11:31:28
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,988
- Website
Re: Sodium-Iron battery?
Aquion Energy are able to make a battery work using salt water: Aquion Energy - Technology It seems they use intercalation to store both ions, rather than chemical reactions. They've managed to make it work, so...
Use what is abundant and build to last
Offline
Like button can go here
#17 2020-02-08 19:31:42
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,917
Re: Sodium-Iron battery?
4 topics that meantion this:
Nonflammable electrolyte for high-performance potassium batteries
This novel electrolyte contained triethyl phosphate as the sole component of the solvent. This substance is known as a flame retardant. It has been tested in lithium-ion batteries, but only very high concentrations provided enough stability for long-term operation, too high for industrial applications.
The battery industry demands dilute electrolytes, which are cheaper and ensure better performances. They combined the phosphate solvent with a commonly available potassium salt and obtained an electrolyte that did not burn and allowed stable cycling of the assembled battery concentrations of 0.9 to 2 moles per liter, which are concentrations that are suitable for larger scales; for example, in smart-grid applications.
Offline
Like button can go here
#18 2021-07-26 20:23:44
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,917
Re: Sodium-Iron battery?
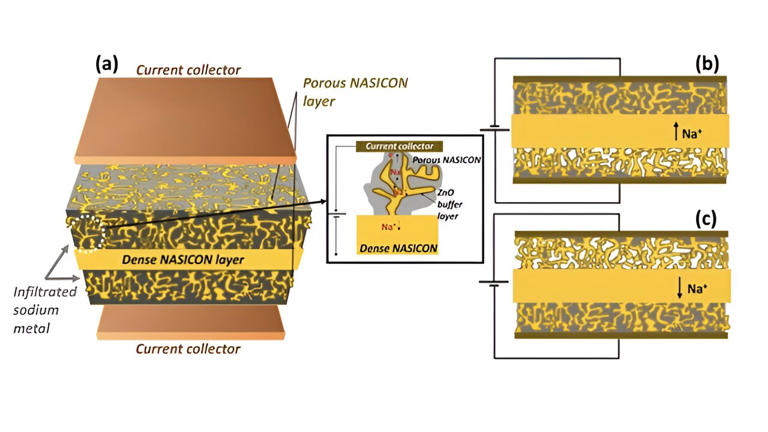
another battery of iron....
Offline
Like button can go here
#19 2021-07-27 02:10:20
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,269
Re: Sodium-Iron battery?
I'm not sure the batteries operation would actually involve metallic sodium. It depends on how the sodium is trapped in the anode. Is it kept in metallic form, or as positive ions in a negatively charged structure? If the latter, there shouldn't be a problem.
The impression I get from the KurzweilAI article is that the cellulose anodes store them as ions, so it should be okay.
It has been a couple of decades since I studied chemistry. But from what I remember, sodium has only two oxidation states, either zero or +1. Iron and nickel are transition metals and have variable oxidation states. Hence, sodium must be included as a metallic substance, as its oxidation state must change for it to produce energy. The same with lithium.
The obvious advantage that sodium and iron have over lithium are their abundance. They are both amongst the most common elements in the Earth's crust. Sea water contains sodium in effectively infinite quantities as far as humans are concerned.
Last edited by Calliban (2021-07-27 02:15:12)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#20 2023-12-20 15:02:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,917
Offline
Like button can go here