New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2015-11-30 19:48:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Regolith processing to create oxygen only
This topic is to help in the measuring of progess to build equipment needed to process regolith to make oxygen from insitu resources.
http://sbir.gsfc.nasa.gov/SBIR/abstract … -8819.html
http://ntrs.nasa.gov/archive/nasa/casi. … 012503.pdf
http://isru.nasa.gov/Molten_Regolith_Electrolysis.html
http://ntrs.nasa.gov/archive/nasa/casi. … 003037.pdf
lets analyze the methods to produce oxygen, the mass required to make it happen and the energy needs to make it all work.
This was a quick search are there other methods to reference?
Offline
Like button can go here
#2 2015-11-30 20:17:31
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,065
Re: Regolith processing to create oxygen only
I would prefer to head in this direction. (Get it out of the Atmosphere on Mars).
http://newmars.com/forums/viewtopic.php … 80#p126780
As I recently read from another, it is much easier to deal with fluids (Air) or fluid like materials (Dunes), then to do a pick and shovel in the hardpans of Mars. But technically dunes and loose soils are Regolith, so you win too.
Last edited by Void (2015-11-30 20:19:08)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#3 2015-11-30 20:50:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Re: Regolith processing to create oxygen only
Yes but we already know how to do liquification of C02 and electrolysis of it to break it down into Co + O
That can be its own seperate topic and has been discussed in the past in lots of other places on the forum.
Offline
Like button can go here
#4 2015-12-01 14:12:17
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,065
Re: Regolith processing to create oxygen only
Yes, fair enough, that was fairly ungenerous of me. The best I can offer on the fly is that it seems possible that ancient Mars had a transitional phase where it might have been rather Oxidized, and even had Oxygen of significance in it's atmosphere, and so, rocks from that presumed era may have an excess of Oxygen.
What I have read about using bacterial to release some of the Oxygen suggests that if you give them Iron of a correct sort, and perhaps sugar, they might release Oxygen for you. Where to get the iron and sugar from however? That involves effort.
As in the Moon, there might be some rocks you could heat up with concentrated solar energy which might release Oxygen.
Last edited by Void (2015-12-01 14:15:30)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#5 2015-12-01 22:59:15
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Re: Regolith processing to create oxygen only
Another way to get oxygen from the regolith does require another step in that when we hydrogenate the regolith in a chamber it will produce some water that with heat can be driven out of the mixture to be cooled then processed by electrolysis. Sure we could make use of solar produced energy for the heat via concentration and electrical power by PV cells. Natural Mars temperature can be used to cool the water vapor driven out of the reduction of the regolith. Of course natural occuring water once processed gets the Oxygen at a lower energy required level but thats been already thought of else where.
http://isru.nasa.gov/Hydrogen-Reduction … olith.html
Another method would be regolith selection of high hydrogen conten to be mixed with regolith with a high oxidation level to combine and heat to cause water to form.
http://www.lpl.arizona.edu/~umpire/prof … 3722v1.pdf
Co versus hydrogen to reduce FeO3
http://www.marspapers.org/papers/Moss_2006_2.pdf
http://www.planetary.brown.edu/pdfs/5032.pdf
nuclear heated Co2 used to heat regolith to drive out water
http://sbir.gsfc.nasa.gov/SBIR/abstract … -8174.html
Offline
Like button can go here
#6 2021-12-04 12:57:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Re: Regolith processing to create oxygen only
could this be the last process in the regolith harvesting for water?
where we electrolysis a bit of the water to make use of the hydrogen to get more?
Offline
Like button can go here
#7 2022-03-12 14:37:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Re: Regolith processing to create oxygen only
Scientists want to farm oxygen from the Martian soil
https://www.digitaltrends.com/news/oxyg … tian-soil/
Offline
Like button can go here
#8 2022-03-12 14:41:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,872
Re: Regolith processing to create oxygen only
“The project, supported through ESA’s Technology Development Element, will include the initial design of a large scale reactor device to periodically extract oxygen from soil, what we term ‘oxygen farming’. Solar UV irradiation will then replenish their oxygen supply within a matter of hours. The estimate is that a 1.2 hectare (3 acre) area would yield enough oxygen to keep a single astronaut alive.”
https://www.esa.int/Enabling_Support/Sp … en_farming
Viking’s ‘Labeled Release’ experiment applied micro-nutrient liquid to a Martian soil sample, which released copious amounts of oxygen in response. Some authorities interpreted this result as evidence of microbial life on Mars – except that even after the sample was sterilised with 160°C heat this oxygen production continued. Meanwhile other Viking experiments found no traces of organic chemicals.
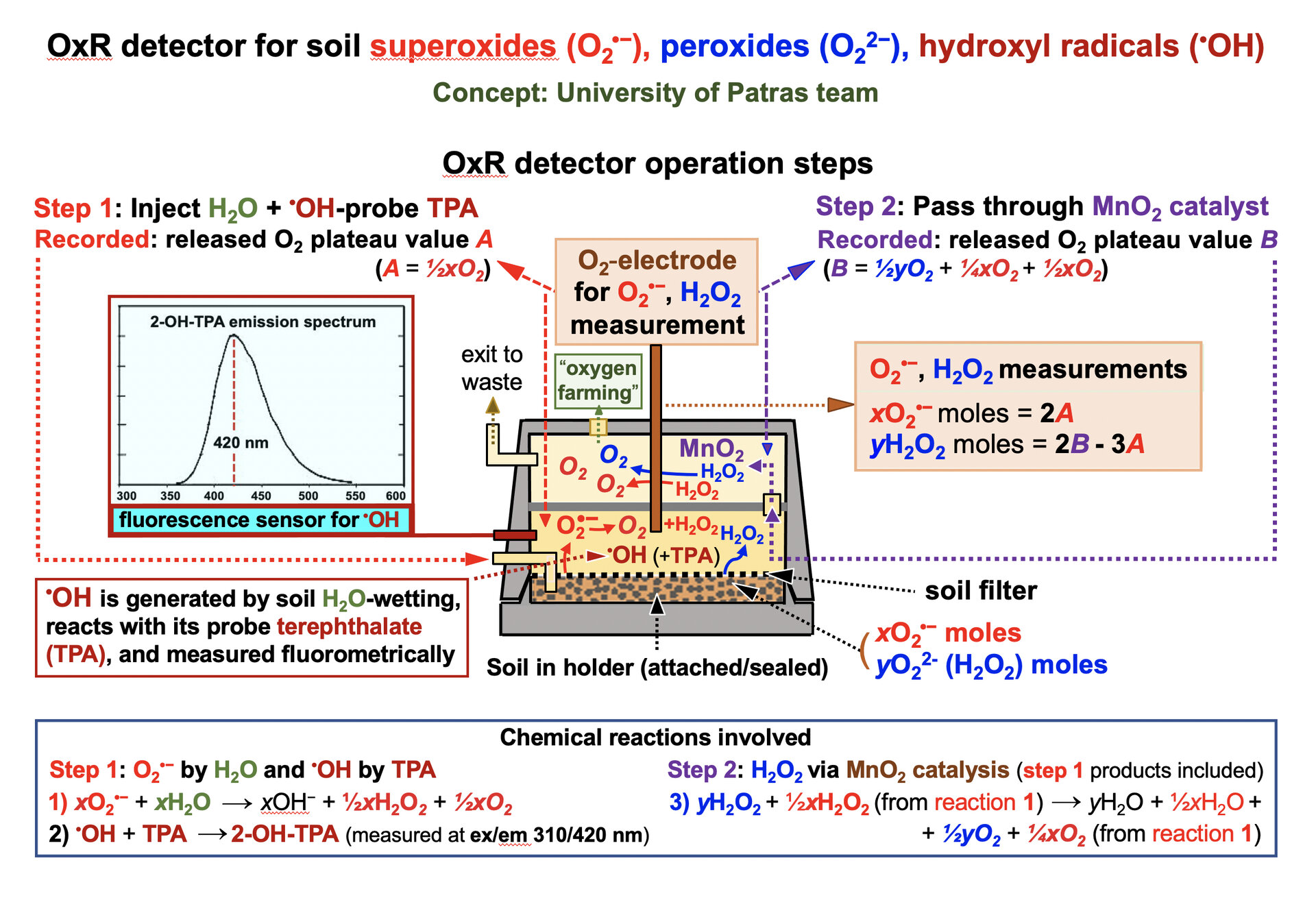
https://www.esa.int/ESA_Multimedia/Imag … or_concept

https://agupubs.onlinelibrary.wiley.com … 07JE003001
The formation and stability of the superoxide radical (O2−) on rock-forming minerals: Band gaps, hydroxylation state, and implications for Mars oxidant chemistry
seems that once you put it back down on the ground after heating for the oxygen and water removal process that it will fill back up in a short period of time due to the high UV levels.
Offline
Like button can go here