New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2008-04-11 20:48:44
- Gregori
- Member
- From: Baile Atha Cliath, Eireann
- Registered: 2008-01-13
- Posts: 297
Re: Impractical way to cool Venusian Atmosphere.
The thick atmosphere of Venus and high Wind speeds make sure that the night side of the planet is as hot as the day side.
Most of the Heat is being transported by the atmosphere. If you could disrupt this flow of heat from the day side to the night, you could potentially make the night side much colder.
I propose an Atmospheric dam around the planet to disrupt the flow. It would probably have to be around 40 km high and encircle the planet from pole to pole. It could be essentially a hollow structure if the materials and design where strong enough. A very wide base to cope with the load would be a preferable.
Offline
Like button can go here
#2 2008-04-13 11:10:36
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,994
- Website
Re: Impractical way to cool Venusian Atmosphere.
True, very impractical.
Use what is abundant and build to last
Offline
Like button can go here
#3 2008-04-15 17:38:30
- Gregori
- Member
- From: Baile Atha Cliath, Eireann
- Registered: 2008-01-13
- Posts: 297
Re: Impractical way to cool Venusian Atmosphere.
yeah, I don't feel too bad since every method of terraforming Venus is impractical.
Has anybody considered settlement on the Venus surface using refractory material and an active cooling system?
There have been submarines that have withstood much greater pressures than the Venusian surface, Materials that can withstand much greater heat and forms of powered cooling. An installation on the surface could be used to exploit the ground beneath it if there are useful minerals beneath.
Actually does anybody know of the possibility of using wind power on Venus?
Offline
Like button can go here
#4 2008-04-18 20:32:20
- Spaniard
- Member
- From: Spain
- Registered: 2008-04-18
- Posts: 144
Re: Impractical way to cool Venusian Atmosphere.
My way
First, make a group of robots with replication capability to the asteroid belt.
Make propulsors with fusion energy enough powerfull to move asteroids to go to Venus and put it in orbit.
Make a lot of gas ballons (with helium obtained from fusion energy used to propulsion and the work of robots) to put it in high elevation, where the temperatures are no enought to damage them.
Make so much ballons that covered a lot of the planet. The ballons will be coated with a high reflection material (like aluminium) that should make Venus to reflect a lot of solar light into space.
This should lower the surface temperature. I hope enought to make robots work permanently on the surface (perhaps only on the poles). I hope that
The darkness would not be a problem because the machines used other form of energy (fusion).
The robots would construct on surface factories to make more robots and make carbon absorbers. This machines will release a lot of oxigen. Carbon will be "secuestrated" converting into a solid and stable material, like nanotubes or diamons.
This tecnology is now available, but is very energy intensive. With fusion, this would not be a problem.
An atmosphere with Earth like temperatures and a lot of oxigen, should be destroy the sulfuric acid.
If not exist enough nitrogen, it could be imported from the moons of Jupiter or Saturn. Another alternative, if advanced fusion was ready, it could be transmutate carbon into nitrogen throught protium-carbon fusion as in stars (far away from current technology, so import is the first alternative).
Oxigen+nitrogen+CO2 extraction+ballons = Cold planet
With enought CO2 removed, some ballons could be removed and return partial light to Venus.
In last place, we could import a lot of water from Kuiper belt.
Offline
Like button can go here
#5 2008-04-18 22:01:01
- noosfractal
- Member
- From: Biosphere 1
- Registered: 2005-10-04
- Posts: 824
- Website
Re: Impractical way to cool Venusian Atmosphere.
Make so much ballons that covered a lot of the planet. The ballons will be coated with a high reflection material (like aluminium) that should make Venus to reflect a lot of solar light into space.
Similar ideas have been discussed for cooling the Earth ...
Teller et al., "Global Warming and Ice Ages: Prospects For Physics Based Modulation of Global Change," DOE Technical Report, August 1996
http://www.osti.gov/accomplishments/doc … CC0229.pdf
... it may be that a solar shade/scatter cloud at the Venus-Sun L1 point is more efficient long term (it certainly requires less mass).
Fan of [url=http://www.red-oasis.com/]Red Oasis[/url]
Offline
Like button can go here
#6 2008-04-19 14:16:55
- Terraformer
- Member
- From: The Fortunate Isles
- Registered: 2007-08-27
- Posts: 3,994
- Website
Re: Impractical way to cool Venusian Atmosphere.
Would 3 Bars of N2 be enough?
Why use Helium? H2 is much lighter.
Use what is abundant and build to last
Offline
Like button can go here
#7 2012-02-11 16:30:00
- dunwich
- InActive
- From: dunwich
- Registered: 2008-02-18
- Posts: 30
Re: Impractical way to cool Venusian Atmosphere.
releasing supergreenhouse gasses might work. By increasing the temprature the atmosphere would start to boil off
People think dreams aren't real just because they aren't made of matter, of particles. Dreams are real. But they are made of viewpoints, of images, of memories and puns and lost hopes.
Offline
Like button can go here
#8 2012-02-11 17:15:27
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,247
Re: Impractical way to cool Venusian Atmosphere.
Part I:
Suppose the people live in orbit around Venus.
Suppose they can microwave the upper atmosphere, and make it swell.
If It becomes Plasma Then
It may be magnetic
If it is magnetic then
It may be collectible by electromagnetic system, enriching the population in orbit
Else
Let the solar wind drag it off
End If
End If
Perhaps a hollow cylinder 1 Mile (A silly number choice) in diameter, and reaching upwards to reasonable temperatures. Floors in the cylinder, partitioning it. Mixtures of gasses in the partitions, suitable to maintain neutral boyancy as needed. The bottom end of the cylinder anchored to the ground. The top end as a landing pad.
Thus humans present in workshops.
Robots in potected "Sheds" on the surface, where inside the conditions would be improved to make their functioning possible. Robots with new technology for very high temperatures. Mining underground from those sheds, and yes refrigeration. Electric power from wind or orbital microwave.
As always Sulphuric Acid is public enemy #1. Could it be altered chemically, by;
-Laser it from above or
-Microwave it from above or
-Drop chemicals into it or
-Enhance to possiblity of Ozone in it's Oxygen layer by putting something there to remove the Chlorine
?
Also, the winds would be very hard on any structure anchored on the ground, but perhaps if there is any wind it is a powerful energy source to refrigerate robots.
Part II:
From the above method my next attempt would be more cylinders, and then a floating covering over the whole planet, somewhere midway in the atmospheric column, and then once that is compete, begin removing CO2 from the atmosphere above it, and injecting it below, and begin removeing Nitrogen from below to put above. Then add Oxygen to the part above.
Extremely reflective of course.
I am calling upon new technologies to accomplish this of course super strong "Nano Materials".
Note: If it could be done, then this could create a shell in the atmosphere which could be walked on by light robots, and in places by humans. Supposing that it were possible to remove atmosphere by the methods in part I, then continually the atmospheric conditions would change, and the plan would have to adjust to that.
It would depend on the desires of the inhabitants. Perhaps they could simply keep re-enforcing their floating platform, and be satisfied to have a thick CO2 atmosphere below and a N2/O2 atmosphere above it. With reflectance and a lack of cloud cover, eventually that which is below would be cool also. One thing limiting this idea is you could not aford to have much water vapor in the atmosphere above, or it would condense on the night side.
C02 might become a problem as well, if the platform got too cold. That would not be a problem until the atmosphere below the platform cooled off.
This whole notion is of course far fetched, but as far as I can see so are pretty much any other notions of working with Venus. Far fetched does not mean impossible.
**NOTE: I can seldom log on to this place. I log on, and it says I have logged in and then it says I am not logged in. Not today though.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#9 2012-02-11 17:24:32
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,370
- Website
Re: Impractical way to cool Venusian Atmosphere.
Genetically engineer terraforming bacteria. Designed to live their entire life-cycle in the clouds. Anaerobic archaea. These single cell organisms would convert CO2 into polyanhydride. That's a form of plastic, molecular formula [CO2]n where 'n' is many thousand. The organisms would use sunlight for energy (photosynthesis) using retinal as its primary photodye just like halobacteria, instead of chlorophyll like most plants. Chlorophyll requires one atom of magnesium for each molecule, retinal is composed exclusively from carbon, oxygen, hydrogen, and nitrogen: gasses found in the atmosphere. This will convert CO2 from the atmosphere into dust that will drift down and accumulate on the surface.
Once pressure has dropped to the desired level, stop it by seeding the planet with cyanobacteria. Anaerobic organisms do not require oxygen, in fact they're poisoned by oxygen. Cyanobacteria release oxygen, the convert CO2 and water into carbohydrates and oxygen.
Offline
Like button can go here
#10 2012-02-12 04:43:36
- karov
- Member
- From: Bulgaria
- Registered: 2004-06-03
- Posts: 953
Re: Impractical way to cool Venusian Atmosphere.
RobertDyck,
Brilliant!
Now tell us more about the bulk properties of the polymerized CO2, pls.
Obviously we are talking about miles thick layer on the ground.
How stable, under what conditions this stuff is?
Offline
Like button can go here
#11 2012-02-12 09:57:03
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Impractical way to cool Venusian Atmosphere.
Chlorine and the lack of Ozone on Mars and Venus, can this be altered?
http://www.marsdrive.com/forums.aspx?g=posts&t=304
Not sure on the quantites to impart to the Venus upper atmosphere but it would start a chain reaction....
Offline
Like button can go here
#12 2012-02-13 12:39:00
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,370
- Website
Re: Impractical way to cool Venusian Atmosphere.
Basic information on polyanhydride is available from Wikipedia. (click the link)
It has been used for implants to the human body, as a time delay for pharmaceuticals. Polyanhydride made as a co-polymer with a drug is weak at the drug, causing it to break down over several weeks. This releases the drug, and the polyanhydride becomes CO2. The body can exhale that CO2 through the lungs. So this means polyanhydride is safe within the human body. The Wikipedia article lists three different types of polyanhydride, all depend on the other thing that it is co-polymerized with. But I'm saying for Venus, we use pure polyanhydride, not a co-polymer. The articles lists unsaturated polyanhydride as having fewer than the maximum co-polymer groups. The unsaturated form is highly crystalline and insoluble in common organic solvents. I assume this holds true for pure polyanhydride. When it says "crystalline", expect a hard plastic, I expect something like high density polyethylene (HDPE). Polyanhydride is highly hydrophobic, it repels water.
Notice the molecular structure: carbon alternates with oxygen, single bonding each atom. Each carbon also has another oxygen atom double bonded. A double bond is very strong, but that's the side atom, not along the chain. To make this molecule stable it would probably require something strong added to each end, to prevent the chain from being "unzipped". You could add a nitrile, which is a single atom of nitrogen triple bonded to carbon. That carbon atom would have to be bonded to another carbon atom in the [CO2]n polymer rather than an oxygen atom to make the link stable. But decomposition in the presence of water could release cyanide. Besides, although there's more nitrogen in the atmosphere than Earth, we need to reserve that nitrogen for the biosphere. Once you grow a forest and other plants covering the entire planet, all the protein in those plants will require a lot of nitrogen. There is sufficient nitrogen on Venus, but we can't consume it in whatever we use to sequester CO2. Sulphur has two bonds per molecule, like oxygen, so isn't really useful. An easy solution is to add a benzene ring, but that would consume 5 hydrogen atoms, one bond connecting the ring to the polymer chain. With 5 hydrogen atoms at each end, it would consume 5 water molecules per polyanhydride molecule. The point of polyanhydride is to avoid consuming water. Benzene is so strong because the carbon-carbon bonds alternate between single and double bonds. But benzene is one of the known co-polymers, it's known to degrade. It may be strong on its own, but will separate from polyanhydride. A sugar ring has single bonds between carbon atoms, which leaves 2 bonds per carbon. Pentose sugar (5 carbon atoms in a ring) is stronger than hexose sugar, which is why DNA uses ribose. Could we add a 5 carbon ring, with 4 of the carbon atoms double bonded to a single oxygen atom like the polyanhydride? The fifth carbon atom double bonded to a single nitrogen atom, which in turn replaces oxygen for the last [CO2] group in the chain? Cyanide has nitrogen triple bonded with carbon, a double bond is not cyanide. This would still consume nitrogen, but perhaps that could be reduce that with ultra-high molecular weight polyanhydride, which means "n" a very large number of units, perhaps over 100,000. Consuming one molecule of N2 per 100,000 (or more) molecules of CO2 would still leave enough N2 for a biosphere.
Last edited by RobertDyck (2012-02-13 12:49:28)
Offline
Like button can go here
#13 2012-02-13 13:03:50
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,370
- Website
Re: Impractical way to cool Venusian Atmosphere.
Ah, yes, Venus has 0.1–0.6 ppm hydrogen chloride. The chlorine does destroy ozone. But simply reducing temperature should stop forming chlorine gasses. Chlorine would take a long time to leave the atmosphere, but would eventually leave. Once Venus has an oxygen atmosphere, UV will create ozone. UV will also destroy ozone, an equlibrium will be reached. Once all the chlorine is out and once the partial pressure of oxygen is the same as Earth, expect UV that reaches the surface to also be the same as Earth.
Offline
Like button can go here
#14 2012-02-13 20:04:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Impractical way to cool Venusian Atmosphere.
I learn something new every time I read posts from RobertDyck and karov regardless of the topic with this being no exception. The creation of Ozone was a new via UV and yes I googled it.
http://www.ozonesolutions.com/ozone_pro … ature.html
And where it happens...Fifteen to thirty kilometers up in the atmosphere, in the layer called the stratosphere...
http://visibleearth.nasa.gov/view.php?id=53166
The atmosphere is still quite thick even at these hieghts....as compared to the edge of space....
http://enhs.umn.edu/current/5103/uv/fate.html
UV radiation is classified into three bands based on varying wavelengths of light. Ultraviolet A (UVA) spans the electromagnetic spectrum of wavelengths between 320-400nm, ultraviolet B (UVB) spans the spectrum between 290-320nm, and ultraviolet C (UVC) spans the spectrum between 200-290nm. Ozone in the stratosphere completely absorbs UVC and partially absorbs UVB; it does not absorb any UVA.
Last edited by SpaceNut (2012-02-13 20:14:47)
Offline
Like button can go here
#15 2012-02-18 21:42:08
- RobertDyck
- Moderator
- From: Winnipeg, Canada
- Registered: 2002-08-20
- Posts: 8,370
- Website
Re: Impractical way to cool Venusian Atmosphere.
A key issue with terraforming Venus is to get phosphorus into the clouds. If we want genetically engineered anaerobic archaea to convert CO2 into polyanhydride, then the microorganism has to survive. Cell membrates are bi-lipid membranes, composed of Carbon Hydrogen and Oxygen. Protein also has Nitrogen, so those four basic elements are the primary building blocks of life: CHON. Carbon and Oxygen are found in CO2, Hydrogen and Oxygen in water, and Nitrogen in N2 gas. Photosynthesis can use retinal instead of cholophyll, because retinal is composed entirely from CHON while chlorophyll requires one magnesium atom per molecule. Halobacteria use retinal; they're purple instead of green. But all life requires DNA and RNA. Nucleotides are composed of CHON as well, as is ribose, but the backbone of DNA or RNA is phosphate. That requires phosphorus.
The atmosphere of Venus has a lot of gasses, but missing phosphorus. It has:
~96.5% carbon dioxide
~3.5% nitrogen
0.015% sulfur dioxide
0.007% argon
0.002% water vapor
0.001 7% carbon monoxide
0.001 2% helium
0.000 7% neon
trace carbonyl sulfide
trace hydrogen chloride
trace hydrogen fluoride
The surface of Venus is basalt, simular to any other rocky planet including Earth. It's hot with land forms squished flat because the rock has been heated until it's soft and squishy. But it should have as much phosphate minerals as Earth. Apparently sulphur in rocks combines with CO2 to form carbonyl sulphide (COS), which decomposes at higher altitudes to become sulphur dioxide. That combines with water and UV light at the top of clouds to become sulphuric acid (H2SO4). Apparently chlorine and fluorine have a means to be liberated from rock to become a gas. However phosphorus does not.
Phosphorus oxide (P4O6) melts at 23.8°C and boils at 173°C. Phosphine is PH3 with some P2H4, a gas at -88°C, but highly flammable. It's toxic, but since it burns would not remain in an oxygen atmosphere. Phosphorus chloride (PCl3) melts at -112°C and boils at 76°C. All these would be gasses near the surface of Venus, but only phosphine would remain a gas in the clouds. When phosphorus oxide dissolves in water it becomes phosphoric acid (H3PO4), the question is how to get it up to the cloud layer where liquid water droplets exist. Phosphoric acid in water rain drops are an ideal source for single cell organisms. Sulphur is liberated from rock by carbonyl, phosphate also forms a carbonyl. The form normally found on Earth is carbonyl phosphate, which has an ammonia component: CH2NO5P. Unusual things happen in the high pressure, high temperature, and anoxic environment of Venus. Carbonylbisphosphonate does exist, which is two phosphates bonded to one carbonyl, but each phosphate normally has an organic something bonded to it. Could pure Carbonyl bisphosphate (that is 'ate' instead of 'onate') form on Venus? Would this break down to phosphorus oxide, which could not rise to an altitude with a temperature below 173°C? One could hope. If so, how do we get it up to the cloud layer? Could we do something that converts phosphorus oxide to phosphine, then let it combine with water in clouds to form phosphoric acid? How would we do that?
Offline
Like button can go here
#16 2012-03-25 03:31:18
- dunwich
- InActive
- From: dunwich
- Registered: 2008-02-18
- Posts: 30
Re: Impractical way to cool Venusian Atmosphere.
looking at Impractical ways to cool down Venus we could try transforming the heat energy in a other forms. The link below talks abouth a LED that can work at 230% effeciency because it can also use the thermal energy around it. Effectivly it it cools it's surrounding.
See this link: http://www.wired.co.uk/news/archive/201 … cient-leds
Last edited by dunwich (2012-03-25 03:32:23)
People think dreams aren't real just because they aren't made of matter, of particles. Dreams are real. But they are made of viewpoints, of images, of memories and puns and lost hopes.
Offline
Like button can go here
#17 2012-03-28 11:51:39
- undormant
- Banned
- Registered: 2012-03-25
- Posts: 18
Re: Impractical way to cool Venusian Atmosphere.
releasing supergreenhouse gasses might work. By increasing the temprature the atmosphere would start to boil off
This I like, has anyone actually suggested making Venus HOTTER before? All that atmosphere is a problem, I agree getting it so hot it boils off into space would be a much better idea. We could then go about creating an atmosphere we actually want/need.
R ![]()
Offline
Like button can go here
#18 2012-03-29 21:01:52
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,247
Re: Impractical way to cool Venusian Atmosphere.
Thanks for pointing that out. I thought about it and I see merit.
From my point of view, and I say that noone has to agree, but as I see it:
-Greenhouse gasses, and swelled atmospheric column.
-Allowed still is floating habitats, but at a higher altitude, and incidently closer to an orbit, and so more accessable by rocket devices.
-Not forbiden is mining by robots on the surface, but hotter, but any such mining was going to require a whole new type of techonology anyway.
-I also see, that if the L2 point contained a facility, (That is in the shadow of the planet Venus), it might aquire electical power from the solar wind, and might using that power capture some of the atmospheric gasses being swept off of the atmosphere of Venus.
So, there is very little to loose except the planetary platform I suggested. I don't care. I proposed that just to try to be helpful. From the start, I was very concerned about how it would deal with Hurricaine winds anyway.
I like this new idea from others much better. (I did try to add something). Also, for those of you who lack the nice ![]() but I won't waste my time on it. Your loss actually.
but I won't waste my time on it. Your loss actually.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#19 2014-10-15 17:44:13
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Impractical way to cool Venusian Atmosphere.
Its funny how reading other sites remind one of topics discussed elsewhere....
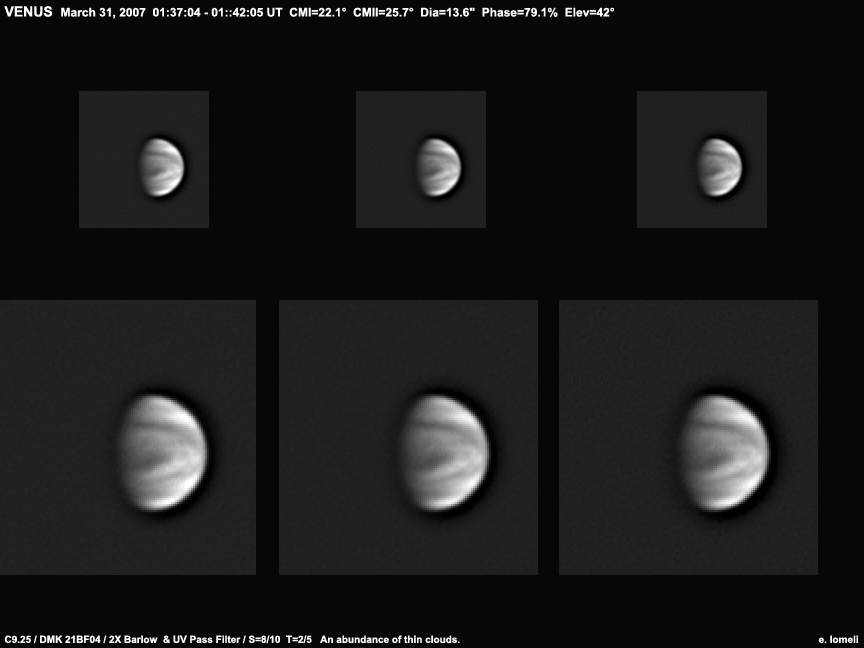
MYSTERY IN THE CLOUDS: Seen through an ordinary telescope, Venus looks a bit dull--it's a featureless, cloud-covered orb. But if that same telescope is fitted with an ultraviolet filter, a mystery reveals itself: Venus' clouds are cross-crossed with rapidly-moving dark bands, shown here in a series of March 30th photos from Ed Lomeli of Sacramento, California:
The bands are the mystery. Some unknown substance within them strongly absorbs UV light, accounting for almost half of the solar energy trapped by Venus. Whatever is in there, it plays a big role in maintaining Venus' hellish climate; the average temperature on the surface is about 460° Celsius. Astronomers have been studying these bands since Mariner 10 first spotted them in the 1970s, but decades later no one knows the identity of the "UV absorbers." Maybe Venus Express, a European spacecraft orbiting Venus now, will eventually solve the mystery.
You can see Venus with your own eyes--indeed you can't miss it. It appears in the western sky at sunset glaring like a landing airplane. A backyard telescope reveals the point of light as a cloud-covered planet, and a UV filter reveals the mystery in the clouds. "I am using a 1.25-inch Schuler photometric UV pass filter. I also have the Baader UV pass filter, but the Schuler is a better performer," advises Lomeli.
http://www.solstation.com/life/ven-life.htm
Four billion years ago, the sun was 40 percent less luminous than today.
Was this Venus that long ago...

If Life Exists on Venus, Could it be Blown to Earth?
We’ve heard about the possibility of extraterrestrial life arriving on Earth from another planet, asteroid or comet, but the mode of transport usually includes a chunk of rock falling through the atmosphere as a meteorite. But there could be another form of interplanetary transportation. What if there are microbial forms of alien life floating in the upper atmosphere of Venus (the planet’s clouds contain compounds that could indicate presence of micro organisms)?
Offline
Like button can go here
#20 2014-10-15 19:23:01
- Tom Kalbfus
- Banned
- Registered: 2006-08-16
- Posts: 4,401
Re: Impractical way to cool Venusian Atmosphere.
All methods of cooling Venus are impractical today, since today we can do none of them! My method is to put a comet at the L1 point between Venus and the Sun, the Comet's tail will shadow Venus, and the Solar Wind and radiation will drive the gases and dust right onto Venus' atmosphere. A comet's coma and tail is way bigger than the actual solid part of the comet. Another idea is simply place the comet in a low orbit just above the planet's atmosphere, I think in that case the dusk kicked off the comet will encompass the entire planet, that is Venus will be within the comet's coma and tail which will reflect some light away from Venus, a similar mechanism can be used to reduce global warming, though hopefully not too much, as we don't want an ice age! I'm not sure I believe in man caused global warming anyway, but blocking some sunlight will cool the Earth or Venus, Venus has a rather more severe case of global warming than the Earth does.
Offline
Like button can go here
#21 2014-10-17 14:02:36
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,247
Re: Impractical way to cool Venusian Atmosphere.
I don't know how stable it would be, but could you build a thin vacuum filled shell world in the L1 zone?
It would not provide as much shade as a comet plume and comet, but it would require less mass shifting. Materials could come from Venus crossing asteroids captured or Mercury.
You would have a very large vacuum filled shell upon which you could attach solar arrays. The interior would have some protection from thermal variables, and would have some protection from micrometeorites.
The volume could contain processing facilities and spinning habitats.
Last edited by Void (2014-10-19 09:31:17)
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#22 2014-10-17 21:09:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Impractical way to cool Venusian Atmosphere.
So what would a skeletal platform for the organism to grow in the Clouds of Venus to keep the leverage point for life and cooling afloat require in its design. Of course the ability to shield and cultivate the organisms growth is a must including Phosphorus being bought to the platform as required along with other such minerals or food for the organism.
Offline
Like button can go here
#23 2019-02-22 16:33:34
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 24,057
Re: Impractical way to cool Venusian Atmosphere.
For Void ...
Some time ago, I was curious to see what your early posts might have looked like.
It will be no surprise to you (of course), but to me, it was striking that the style over 8 years (or so) is so similar.
One refinement I have highlighted below, to try to entice you to say a bit more about the background that inspired this example:
Part I:
Suppose the people live in orbit around Venus.
Suppose they can microwave the upper atmosphere, and make it swell.If It becomes Plasma Then
It may be magnetic
If it is magnetic then
It may be collectible by electromagnetic system, enriching the population in orbit
Else
Let the solar wind drag it off
End If
End If
In your recent exchange with SpaceNut, about 3D Printing, I see an opportunity, if you are willing ...
Among my interests is 3D printing, as I have shown in my response to JoshNH4H, about design of a expanding tower for use on Mars (or Earth for that matter).
Your background (as implied by the code segment above) might include the ability to code Blender to create interesting 3D objects.
I use Shapeways to render my designs, although there are (I believe) plenty of other organizations which provide printing services.
If you are interested in learning Blender, I could use some assistance in getting off the dime to create a model of the pneumatic tower envisioned by JoshNH4H.
(th)
Offline
Like button can go here
#24 2019-02-22 20:13:51
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,247
Re: Impractical way to cool Venusian Atmosphere.
Yes, I have problems with things bubbling up out of me from time to time.
Funny story: I was working at a conglomerate, in a sort of metrology lab / electronics repair / process line troubleshooter / other stuff, and had my feelings hurt by someone, did not want to risk being near that someone anymore, so my boss sent out a E-Mail, saying a job taking care of the lab database and calibration software / programming was open. So on a friday, I sent a reply indicating that I was interested in inquiring about the job. The next Monday at the typical group meeting the boss announced I was it. :<
![]() So, I struggled with it. It involved working with several languages. But I still consider myself a quack. But I think I was innovative. Weathered quite a few very intense audits FDA and such. So, had to dance like it or not.
So, I struggled with it. It involved working with several languages. But I still consider myself a quack. But I think I was innovative. Weathered quite a few very intense audits FDA and such. So, had to dance like it or not.
As, I achieved retirement age, it turned out that a German software company was going to be introduced as the backbone of the company worldwide. It was sort of a "You will do it and you will like it", "And you will do it our way".
I don't know how to goose step properly, so I saw my chance and retired.
I am interested in your offer, already discovered that it is associated with python. I will get back to you.
I really might prefer to buy a Coyote suit and trespass on GW's ranch ![]()
But I will think on it.
Really thanks, I will look it over.
Quack, Quack.
Done
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Offline
Like button can go here
#25 2019-02-22 20:45:05
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,388
Re: Impractical way to cool Venusian Atmosphere.
Blowing away the atmosphere does not cool venus all that much as the heat is not just coming from the sun to which a thinner atmosphere makes higher in level to the surface.

Lossing the atmosphere means we will not be able to teraform venus once we do engineer a means to cause planetary cooling.
https://en.wikipedia.org/wiki/Terraforming_of_Venus
Orbiting screens, filters to lessen the amount of energy getting that venus would recieve.
In another topic that measurement of energy for venus is 2600 watts...so every other meter of incoming energy needs to be blocked at a minimum.
What better way to create an orbiting energy field with solar panels that stay synchronous to the planets hot side.
Another engineering style forum Sun Shade concepts for Venus
Offline
Like button can go here