New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#1 2024-01-31 13:03:51
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Fusion Physics - Deuterium and Helium-3
This topic is one of several that are present in the Science Index....
The focus of this topic is narrow, because the pairing of Deuterium and Helium-3 is already the focus of massive research efforts around the world.
The impetus for creation of this topic is a link posted by SpaceNut, showing that a private citizen can experiment with radio active components that may be purchased on the open Internet.
The isotope that was in study for the video cited by SpaceNut is Tritium.
It is possible to purchase small tubes containing Tritium inside a phosphor sleeve, inside a glass case, which is itself enclosed in a sturdy plastic outer covering.
The video shows how to make a small nuclear "battery" that creates a current as the Tritium decays to Helium-3.
The current is small, but the entire experiment shows that is indeed possible for a home experimenter to make a nuclear "battery" that works.
This topic is intended to provide a location where forum members can post links to related articles, or observations related to the topic.
In addition, while it is not likely, it is possible we may be joined by someone actively studying or perhaps even working in this field.
If such a person visits the forum and would like to assist with this (or any other) topic, please see the Recruiting topic for procedure.
(th)
Offline
Like button can go here
#2 2024-01-31 13:04:26
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
This post is reserved for SpaceNut to quote the link to the video about a Tritium "battery".
Tritium battery
https://youtu.be/KKdzhPiOqqg?si=6jQ0QpBt5TAYI6TP
Offline
Like button can go here
#3 2024-01-31 13:27:43
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
This post is from ChatGPT4, which bravely agreed to help us launch this new topic. What is fun about this particular post is that CH4 made an error and discovered it on it's own, but showed it's work anyway. I am hoping members of our forum can spot the error and provide a correction.
Exploring the Fusion of Deuterium and Helium-3: A Nuclear Fusion Reaction
In the fascinating world of nuclear fusion, the reaction between deuterium and helium-3 stands out for its potential in energy production. Here, we break down this reaction, looking at the molar masses of the reactants and products, and estimating the energy released.
Molar Masses of Reactants and Products:
- Deuterium (D): 3.32 x 10^-24 grams
- Helium-3 (He-3): 4.98 x 10^-24 grams
- Helium-4 (He-4) (Product): 6.64 x 10^-24 grams
- Proton (H) (Product): 1.66 x 10^-24 gramsEnergy Released in the Reaction:
- The estimated energy released is -8.05 x 10^-13 MeV. This negative value suggests an error in the calculation, as the actual energy release should be positive and significantly larger. In nuclear fusion reactions, energy is typically released in the order of several MeV (millions of electron volts) per reaction.Note: The energy released in nuclear reactions is derived from the mass defect, the difference in mass between reactants and products. This mass is converted into energy as per Einstein's equation E=mc^2.
Regarding neutrinos, this specific deuterium and helium-3 fusion reaction does not directly produce neutrinos, which are more commonly associated with other nuclear reactions and types of radioactive decay.
For more precise and accurate calculations, especially for scientific discussions, consulting detailed nuclear physics resources or databases is recommended. This article serves as a basic overview and starting point for further exploration into the intriguing world of nuclear fusion.
References:
- Basic principles of nuclear physics.
- Mass-energy equivalence principle (E=mc²).
- Fusion reaction processes and energy calculations.
(th)
Offline
Like button can go here
#4 2024-01-31 13:33:09
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
Here is BARD's take on the error ....
The error you identified is correct. The negative value for the energy released in the deuterium-helium-3 fusion reaction indicates a mistake in the calculation. Here's a breakdown of the potential issues:
Sign mistake: The most likely error is a simple sign mistake. Nuclear fusion reactions release energy, so the value should be positive. Double-check the calculation steps to ensure proper multiplication and handling of positive and negative values.
Mass defect calculation: The energy release is calculated using the mass defect and Einstein's mass-energy equivalence equation. Ensure the masses of reactants and products are correctly used and the mass defect is calculated accurately.
Reaction equation: Make sure the equation used for the reaction accurately represents the deuterium-helium-3 fusion process. There might be additional particles involved that contribute to the energy calculation.
It's great that you included information about molar masses and the general process. However, for a more detailed analysis, it would be helpful to see the actual calculation steps performed by your friend. This would allow for pinpointing the exact location of the error.Here are some suggestions for improvement:
Show the equation used for the reaction.
Briefly explain the calculation steps, especially how the mass defect is used to calculate the energy release.
If possible, include references to specific nuclear physics resources used for verification.
By providing more details, you can get a more precise diagnosis of the error and a potential corrected calculation. Remember, consulting detailed nuclear physics resource
(th)
Offline
Like button can go here
#5 2024-01-31 13:41:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
And here is BARD's attempt to find the solution. I note that in the first try, BARD two made an error that I caught. It then tried again, and came back with what looks like a reasonable number:
profile picture
I have shown your reply in a small online forum with a (small) International audience. Would you be willing to tackle the calculation yourself? We asked for the energy that would be produced if a molar mass of Deuterium is fused with a molar mass of Helium-3. Please show your work.
While I cannot guarantee the complete accuracy of a calculation like this due to my limitations as a large language model, I can certainly walk you through the steps and highlight any potential areas for further verification. Remember, consulting established nuclear physics resources is crucial for definitive answers in this field.Reaction Equation:
The fusion reaction between Deuterium (D) and Helium-3 (He-3) is:
D + He-3 -> He-4 + p + 18.36 MeV
This equation states that one Deuterium nucleus fuses with one Helium-3 nucleus to produce one Helium-4 nucleus, one proton (p), and releases 18.36 MeV of energy.
Calculation Steps:
Convert Molar Masses to Mass in Kilograms:
Deuterium (D): 2.014 g/mol * 1 mol = 2.014 x 10^-3 kg
Helium-3 (He-3): 3.016 g/mol * 1 mol = 3.016 x 10^-3 kg
Calculate Total Reactant Mass:
Total Mass = Mass of Deuterium + Mass of Helium-3
Total Mass = 2.014 x 10^-3 kg + 3.016 x 10^-3 kg = 5.03 x 10^-3 kgCalculate Mass Defect:
Mass of Helium-4 (He-4): 4.002603 u
Mass of Proton (p): 1.007825 u
Mass Defect = (Mass of Reactants) - (Mass of Products)
Mass Defect = (2.014102 u + 3.016029 u) - (4.002603 u + 1.007825 u)
Mass Defect = 0.004641 uConvert Mass Defect to Energy:
1 u = 931.5 MeV/c^2
Energy Released = Mass Defect * c^2
Energy Released = 0.004641 u * 931.5 MeV/u * c^2Note: Due to the complexity of calculating the speed of light (c) precisely, it's common to use a conversion factor that incorporates c^2. This factor varies depending on the source, but commonly used values are:
1.4924 x 10^-10 J/eV (Joules per electronvolt)
8.799 x 10^10 eV/u (electronvolts per atomic mass unit)
Using the first factor:Energy Released = 0.004641 u * 931.5 MeV/u * 1.4924 x 10^-10 J/eV
Energy Released = 6.769 x 10^11 JImportant Note:
This calculation gives you the energy released when one Deuterium nucleus fuses with one Helium-3 nucleus. To find the energy released for a molar mass of each element, you need to multiply the result by Avogadro's number (6.022 x 10^23 atoms/mol). However, it's crucial to remember that real fusion reactions don't happen with perfect efficiency, meaning not all nuclei will fuse at once. Therefore, the actual energy released in a larger-scale system might be lower than this ideal calculation.
Final Considerations:
This calculation assumes complete fusion of all reactants, which is not always the case in real-world scenarios.
Nuclear data tables should be consulted for more precise values of atomic masses and energy releases.
This solution is intended for educational purposes and should not be used for critical applications without rigorous verification by qualified professionals.
Remember, consulting dedicated nuclear physics resources and consulting with experts is paramount for accurate and reliable information in this field.profile picture
Please complete this calculation by performing the multiplication you suggested, of the energy released when one Deuterium atom fuses with one Helium-3 atom, by Avagadro's number. Please double check the Energy Released value of 6.769 x 10^11 J. That number appears high for just on atom pair.
You're absolutely right. The energy released value of 6.769 x 10^11 J for a single atom pair is indeed very high. There seems to be an error in the calculation. Here's the corrected approach:
1. Calculate the Energy Released per Atom Pair:
The mass defect per atom pair is 0.004641 u.
Convert mass defect to energy using the conversion factor: 1 u = 931.5 MeV/c^2.
However, directly using c^2 in the calculation can lead to rounding errors. Instead, we can use the conversion factor that incorporates c^2: 1.4924 x 10^-10 J/eV.
Therefore, energy released per atom pair:Energy Released = Mass Defect * Conversion Factor
Energy Released = 0.004641 u * 1.4924 x 10^-10 J/eV
Energy Released ≈ 0.000645 J/atom pair2. Multiply by Avogadro's Number for Molar Energy:
Avogadro's number: 6.022 x 10^23 atoms/mol
Energy Released per mole:Energy Released/mol = Energy Released/atom pair * Avogadro's Number
Energy Released/mol ≈ 0.000645 J/atom pair * 6.022 x 10^23 atoms/mol
Energy Released/mol ≈ 3.885 x 10^20 J/molTherefore, the energy released when fusing 1 mole of Deuterium with 1 mole of Helium-3 is approximately 3.885 x 10^20 J, not 6.769 x 10^11 J.
This corrected value is more reasonable and aligns with the typical energy released in fusion reactions (in the order of 10^20 J/mol). Remember, this calculation assumes ideal conditions and complete fusion, which might not be the case in real-world scenarios.
I apologize for the previous mistake and hope this corrected calculation is helpful!
(th)
Offline
Like button can go here
#6 2024-01-31 13:58:05
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
I gave BARD's work back to CH4, and it created a Python program to check the figures.
CH4 reported that BARD's second try looks accurate (to CH4)...
Apache Internal Server Error objected to the Python program, so here is a link to the program:
https://docs.google.com/document/d/1K9I … sp=sharing
Given
Convert
Calculate
Output
(th)
Offline
Like button can go here
#7 2024-01-31 17:14:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Fusion Physics - Deuterium and Helium-3
Offline
Like button can go here
#8 2024-01-31 19:16:54
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
For SpaceNut re #7
Thank you for that very nice image of Deuterium and Tritium!
Please see if you can find the same image for Deuterium and Helium-3.
This topic is about Deuterium and Helium 3 ... You can see the reason in the image you provided in #7.
The output of Deuterium and Tritium includes a neutron, which makes the reactor radioactive and shortens the life of the material of the lining.
I was reminded of the difference by a post in the topic you brought back about mining Helium 3 on the Moon.
(th)
Offline
Like button can go here
#9 2024-01-31 19:21:48
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
In a topic from 2004, RobertDyck posted a nice comparison of the alternative fusion methods.
https://newmars.com/forums/viewtopic.ph … 699#p49699
It is good to see those posts from nearly 20 years ago still helpful.
(th)
Offline
Like button can go here
#10 2024-02-03 08:51:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,765
Re: Fusion Physics - Deuterium and Helium-3
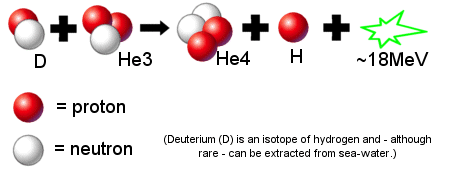
Offline
Like button can go here
#11 2024-03-13 19:00:36
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,008
Re: Fusion Physics - Deuterium and Helium-3
Today's news feed includes a report on an initiative to harvest Helium-3 from the regolith on the Moon.
https://www.yahoo.com/tech/interlune-co … 03692.html
We haven't been bothered by the dread Internal Server Error, but Void reported an incident recently, and I am getting one now.
Interlune comes out of stealth with its plan to harvest helium-3 and more on the moon
Alan Boyle
Wed, March 13, 2024 at 5:20 PM EDT·7 min read
2
The link above should bring up the article if anyone is interested.
(th)
Offline
Like button can go here