New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#501 Re: Planetary transportation » New idea for Mechanical CounterPressure suit » 2015-08-09 08:51:30
The heat of combination of C and O2 is given by: C(graphite)+ O2(g)= CO2 (g) Standard Enthalpy Reaction= -393.5 kJ/mol . Since the molecular weight of O2 is 32, this means in this reaction 32 grams of O2 would result in 393,500 joules of energy being released. So 393,500/32 = 12,300 joules per gram of O2, or 12.3 megajoules per kilo of O2. So reversing this reaction you would need to supply 12.3 megajoules of energy to chemically separate 1 kilo of O2 from CO2.
This is less energy than for water at 18 megajoules per kilo of O2. However, you would still have the problem of the high amount of power needed to compress the O2 to the pressure needed by humans.
Bob Clark
#502 Re: Planetary transportation » New idea for Mechanical CounterPressure suit » 2015-08-08 20:04:23
...
One interesting feature of atmosphere harvesting is self-pressurization. Robert Zubrin's "Mars Atmosphere Carbon Dioxide Freezer" would operate at night because temperature was so low it only takes a few degrees to freeze out dry ice. Then seal the canister and heat to convert into CO2 gas. The phase change will self-pressurize. Your sorbent to collect O2 from Mars atmosphere might have the same property. Thermal release of O2 might self-pressurize. This is important because pumps to go from 7 mbar to Habitat pressure will consume a lot of power. My design to produce diluent gas required pressurizing to 10 bar while freezing out CO2. Going from 7 mbar to 10 bar requires a LOT of power. That's the catch. If you can avoid that, you may have something good.
I ran some numbers on another forum about replacing scuba tanks. It should be doable technically to do electrolysis to separate the oxygen out of water at a much lighter weight than a scuba tank, which run about 15 kilos for about an hour's worth of breathing time underwater.
To chemically separate the hydrogen and oxygen in water takes about 16 million joules of energy per kilo of water. This is at 100% efficiency. I don't know what the best efficiency actually is now but we'll see the amount that can done be using batteries is so high that likely it can be better than using scuba tanks for the same weight.
The ratio of oxygen to hydrogen in water by mass is 8 to 1. So using 16 megajoules of energy you get 8/9 of a kilo of oxygen. This means 16*(9/8) = 18 megajoules gives 1 kg of oxygen. Using as an estimate that oxygen usage for astronauts would be similar to scuba divers we need .5 kg of O2 for 7 hours underwater, or 1 kg of O2 for 14 hours.
Now for the weight of batteries. See this table for a list of energy densities per weight:
https://en.wikipedia.org/wiki/Energy_de … materialsy
The best energy density among the commonly available batteries is the lithium battery (non-rechargeable) at 1.8 megajoules per kilo. Then to provide the 18 megajoules to get 1 kilo of oxygen you would need 18/1.8 = 10 kilos of the lithium batteries. I'll assume the weight of the system will be dominated by the weight of the batteries since electrolysis can be done simply by placing electrified wires in the water. So at 15 kilos of the batteries, the same weight as the scuba tank for 1 hour of breathing time, you would actually get enough oxygen for (15/10)*14 = 21 hours. Or said another way, to get enough oxygen for 1 hour would only take 10/14 = .7 kilos, about 1.6 pounds.
(Note: Edited calculations.)
Practical problems of course are for one how efficient is the electrolysis procedure? Also I believe the lithium batteries catch fire when wet. You would need to insure the battery casings are super-waterproof.

Then there is the weight of the electrolysis components, and the weight of the tank to hold the oxygen as it is being produced. You would also need to dispense with the hydrogen in a way to insure it does not combine with the oxygen to catch fire. It might be sufficient for this to mix it with surrounding water.
Though this would be technically doable the biggest problem is that scuba tanks do not use pure oxygen because it is toxic at depth. Perhaps GW can answer a question on this. The ISS astronauts would not need to prebreathe if their suits were at .8 bar O2. If O2 was given to divers at this pressure could it be used at depth without toxic effects?
This method could also be used to replace the heavy compressed oxygen tanks in astronauts suits by just carrying the small amount of water instead. The Primary Life Support System used on the astronaut spacesuits weighs 38 kilos. The power to separate out the approximate .54 kg oxygen out of water would only take about 5 kg of lithium batteries.
However, for the Mars spacesuit case, it might be more advantageous to separate out the O2 from the CO2 atmosphere. How much power is needed to separate 1 kg of O2 out of CO2? If the power required is not too high you may even be able to do it with solar cells so the astronauts could spend virtually unlimited amounts of time on the surface in their suits.
Bob Clark
#503 Re: Planetary transportation » New idea for Mechanical CounterPressure suit » 2015-08-08 12:57:15
The Popular Science article links to a science paper published by Chemical Science. here Quoting that paper...
Oxygen desorption for the parent [{(bpbp)Co2III(O2)}2(bdc)]4+, crystallized with four different counteranions, was measured by heating up to 160 °C at 5° min-1 under a steady flow of N2 gas.
...
At approximately 100 °C the nitrate salt has lost both O2 molecules, whereas the PF6- salt is completely deoxygenated first at 160 °C.Figure 2 of that paper shows thermal cycling from +30°C to +140°C. How much energy for that thermal cycling? What is the specific heat for this material? What mass of material per unit mass of oxygen? Then there's energy efficiency: is the material compatible with radio frequency heating, like a microwave oven? If you use electro-resistive heating, there's thermal mass to the heating element and container.
Thanks for that. It would definitely take more power to effect the oxygen release than to collect the air through a fan.
Bob Clark
#504 Re: Planetary transportation » New idea for Mechanical CounterPressure suit » 2015-08-07 13:20:23
It occurs to me a bigger energy cost will be involved in increasing the tiny oxygen partial pressure to that used in NASA spacesuits, ca. 0.3 bar. I'll look up some formulas for that.
Bob Clark
#505 Re: Planetary transportation » New idea for Mechanical CounterPressure suit » 2015-08-07 11:20:05
RGClark wrote:Weird Crystal Can Absorb All The Oxygen In A Room -- And Then Release It Later.
Popular Science
This could potentially make fuel cells, space travel, and scuba diving a lot more efficient.
http://www.popsci.com/article/science/w … m9tRRgO.30The Martian atmosphere contains a small percentage of oxygen:
Atmosphere of Mars.
Chemical species Mole fraction[1]
Carbon dioxide 96.0%
Argon 1.9%
Nitrogen 1.9%
Oxygen 0.145%
Carbon monoxide 0.0557%
http://en.wikipedia.org/wiki/Atmosphere_of_MarsWhile this is a low percentage, it's a much higher percentage than that of CO2 in the Earth's atmosphere on which all plant life on Earth is dependent, and therefore on which all animal life is also dependent.
Because of the presence of free O2 in Mars atmosphere, conceivably you could have unlimited spacesuit time on Mars with the oxygen drawn from the surrounding atmosphere. However, the amount of O2 on Mars is so small you would need a fan to draw in more atmosphere. How much air flow would there have to be to get the required amount of breathable oxygen for a person?
I can make an estimate based on what NASA uses on their spacesuits. According to this page, with recycling, 0.54 kg of oxygen is enough for 7 hours of EVA:
The Primary and Secondary Life-Support Systems.
http://quest.nasa.gov/space/teachers/suited/5emu4.html
Let's see how much oxygen is in the Mars atmosphere as a mass percentage. To convert from a mole fraction to mass fraction you multiply the mole fraction times the molecular weight to get a mass in grams. Then the mass fraction for a component is the fraction of this mass divided by the sum of the masses of all the components. This gives a mass fraction of oxygen of about 1/1000 for Mars. For Mars atmospheric density 1/100th of Earths, this is 1.2 /(100*1,000) = 1.2*10^-5 kg/m^3.
At an oxygen usage rate of .54 kg for 7 hours, this is .54/(7*60) = 1.28*10^-3 kg per minute. So you would need about 100 cubic meters of Mars air flow per minute. Say your life support backpack had a 1 square meter area. This would require your fan to move the Martian air at about 100 meters per minute, 1.67 m/s, though the backpack.
Because of the low density of the Martian air this takes surprisingly low power. See this page for the power generated by a wind mill:
Power generated from the wind.
The theoretically available power in the wind can be expressed as
P = 1/2 ρ A v^3 (1)
where
P = power (W)
ρ = density of air (kg/m^3)
A = area wind passing through perpendicular to the wind (m^2)
v = wind velocity (m/s)
http://www.engineeringtoolbox.com/wind- … _1214.html
Assume that operated in reverse the amount of power input will generate the corresponding amount of air flow. Then the power needed for 1.67 m/s airflow speed at an atmospheric density of .012 kg/m^3 and area of 1 m^2 would be P = .5*.012*1*1.67^3 = .028 watts. Even at 20% efficiency this would be about .14 watts of power.
Bob Clark
#506 Re: Human missions » Inflatable space elevator gets a lift » 2015-08-06 09:22:21
Thanks. Pretty cool.
Bob Clark
#507 Re: Unmanned probes » Dawn - Vesta & Ceres orbiter » 2015-08-06 09:20:42
Just released today:
Tour Weird Ceres: Bright Spots and a Pyramid-Shaped Mountain.
http://www.youtube.com/watch?v=Inc9BtRip04
Bob Clark
#508 Re: Unmanned probes » Low cost Europa lander missions. » 2015-07-27 08:34:23
The New Horizons mission to Pluto further confirms the great interest the public has for planetary missions. The Mars Curiosity mission got over a billion hits to the NASA web page over a year. And New Horizons mission web page got 10 million hits on July 14th alone.
A lander mission to Europa to explore the subsurface ocean could result in the most revolutionary scientific discovery in human history: the discovery of life on another world. Such a discovery would dwarf even the Apollo missions in importance.
Then it is notable that following the commercial space approach such a mission could be privately financed at the few hundred million dollars range. This would be low enough considering the great interest such a mission would get that it could even be profitable from advertising.
Then I have changed my sig file to indicate such a revolutionary mission could be accomplished at such low cost and in a near time frame.
Bob Clark
#509 Re: Human missions » A Return to the Moon by the Apollo 11 50th Anniversary. » 2015-07-06 07:31:13
A recent report suggests using the hydrogen tank of an upper stage for the SLS as a space station:
Skylab II: A NASA 'Back to the Future' Concept to Open Up Space Exploration
By Mark Whittington | Yahoo! Contributor Network – Fri, Dec 21, 2012
http://news.yahoo.com/skylab-ii-nasa-ba … 00842.html
(expired link. Try this instead:NASA Mega-Rocket Could Lead to Skylab 2 Deep Space Station.
"Living beyond the moon
Griffin and his colleagues envision placing Skylab II at the Earth-moon Lagrange point 2, a gravitationally stable location beyond the moon's far side.
Over the past year or so, NASA has been drawing up plans for a possible manned outpost at EM-L2. A station there would establish a human presence in deep space, serve as a staging ground for lunar operations and help build momentum for exploring more distant destinations, such as asteroids and Mars, advocates say.
The Skylab II concept could also help ferry astronauts to these far-flung locales, Griffin said."
by Mike Wall, Space.com Senior Writer | April 02, 2013 07:30am ET
http://www.space.com/20444-nasa-deep-sp … ylab2.html )Note there had been suggestions before of using the space shuttle external tank(ET) as a space station:
The Space Island Project
http://www.youtube.com/watch?v=sYIo-0qo9FASTS External Tank Station
www.astronautix.com/craft/stsation.htmThe External Tank Torus.
A Technical Review by David Buth
http://freemars.org/studies/torus/ettoru2.htmlUsing the External Tank From the Space Shuttle as a Space Station ...
aeromaster.tripod.com/grp.htmAt an empty tank mass of 26.5 metric tons(mT) this would be well within the
capability of the 70 mT SLS of getting this to LEO, as at least an outer hull
of a space station. Note for this purpose we could remove the ET bulkheads so
it would even weigh less than this.
This would have a two and a half times the volume of the ISS.
And at the 130 mT payload capacity of the later SLS version, using Centaur
style in-space stages we could even transport this to the Moon.
That "Skylab II" idea was for using just the hydrogen tank only of a proposed upper stage for the SLS. But the entire tank for the SLS core, 50% large than the shuttle ET, will have an approx. 3,000 cubic meter volume. This is over 3 times the pressurized volume of the ISS and over 6 times that of the ISS habitable volume:
International Space Station: By the Numbers.
by Remy Melina | August 03, 2010 04:49pm ET
"1.5: The number of 747s that would provide the equivalent volume of space that is pressurized within the ISS, allowing the crew to work without spacesuits. Of the 33,023 cubic feet (935 cubic meters) of pressurized volume, about 15,000 (425 cubic meters) is habitable volume where astronauts can live ? more room than a conventional three-bedroom house."
http://www.space.com/8876-international … mbers.html
Note that the 70 metric ton payload capacity of the Block 1 SLS was without an upper stage. So the core plus 70 mT payload would make it to orbit. So it could be used as a space station or as a propellant depot in LEO.
But that "Skylab II" proposal was as a station at L2. The problem would be getting the core stage's 85 metric dry mass to L2. With the 70 mT payload capacity to L2 likely a SEP stage could take the core to L2 considering the lowered mass needed for solar electric propulsion. Once there it could also be used as a propellant depot.
It would seem that the SLS itself could be used to place it at L2 if refueled in LEO. Note it would only need a fraction of the ca. 1,000 mT propellant load of the SLS for the purpose, perhaps only about 100 mT. Unfortunately the SSME's are not restartable. They are reusable but they require significant refurbishing before they are fired again.
I have discussed before that with large chemical rocket stages placed at L2, such as the SLS core, that they could be used to cut the transit times to weeks instead of months just using chemical propulsion alone. The problem with using the SLS core for the purpose is that the SSME's are not restartable without refurbishment. It may be possible if there is a station already set up at L2 then the required refurbishment could be done there.
Bob Clark
#510 Re: Human missions » International Space Station (ISS / Alpha) » 2015-07-01 23:14:31
The July 3 Russian Progress Cargo Launch Will Help ISS, But Not Much as mission will be carrying containers of water
http://www.spaceflightinsider.com/organ … r-the-iss/
The spacecraft will deliver more than 3 tons of food, fuel and supplies to the crew and dock to the Pirs docking compartment after a two-day ride.
According to the spokesperson it would be difficult to add extra cargo to the Progress ship as it could delay the launch. Any addition of cargo would have been part of late-cargo loading on the Progress which is possible until about 24 hours prior to launch.
The title is misleading since a NASA official is quoted as saying the station keeps a six-month leeway in needing supplies.
Bob Clark
#511 Re: Human missions » Yet another Mars architecture » 2015-06-24 07:03:55
We actually had a topic with Depot's but it is so dependant on type of fuel that is being stored as to its usefulness...
Here is yet another architecture... A Scenario for a Human Mission to Mars Orbit in the 2030s
Its from Hoppy Price*, John Baker*, and Firouz Naderi* with thoughts Toward an Executable Program Fitting Together Puzzle Pieces & Building Blocks, A Future In-Space Operations (FISO) Telecon May 20, 2015 by *Jet Propulsion Laboratory California Institute of Technology.It is suggested that splitting the efforts into a 2 step plan of first going to Mars orbit for a cycle duration and then a short stay on the surface in a later mission that returns to orbiting base as set by the first mission finally followed by a long term stay on the surface is to there liking. pg7/8
Don't know if the report discusses this but it has been suggested that Phobos has high amounts of subsurface water as Mars does. This conclusion comes from its surprisingly low density. Then it may be possible to set up propellant depots on Phobos and you could do the Mars landing on the first mission. Note also having a propellant depot on Phobos allows you to do a fully propulsive landing on Mars, solving the problem of landing large mass on Mars.
Bob Clark
#512 Re: Human missions » Yet another Mars architecture » 2015-06-20 15:00:49
RobS wrote:Regarding the radiation issue, nuclear thermal doesn't help. If you go to Mars faster, you can't aerobrake; the atmosphere isn't thick enough (according to Zubrin) if transit times are less than 5 months. So if you go faster, you have to haul along the fuel to stop, then the fuel to return to Earth faster as well. It's simply not feasible, from everything I have read.
If you have unlimited propellant such as from lunar-derived propellant depots that's not a problem with either nuclear propulsion or chemical propulsion. I prefer chemical propulsion since it's already here.
After a calculation I was surprised to discover that with orbital propellant depots, manned interplanetary flights become scarcely more expensive than flights to LEO. That is, spaceflight to the Moon, Mars, Venus, asteroids could become as routine as flights to the ISS are now just by having propellant depots in place at departure and arrival points.
For instance rather than the 1,000 metric tons(mT) estimated needed to be launched to orbit for a manned Mars mission, a single empty Falcon 9 first stage at ca. 15 mT dry mass could do ALL the propulsion stages by itself, when you have propellant depots at Earth orbit and Mars orbit. No huge, and hugely expensive, Mars Colonial Transport, SLS, or even Falcon Heavy required.
And it's not just the Falcon 9 first stage. It would also work for the first stages for the Atlas V, the Delta IV, the Ariane 5, the Soyuz, etc., with the empty stages lofted to orbit and refueled in orbit at both departure and arrival points. This means every space faring nation could do Mars missions for little more than currently used to send flights to the ISS.
Here is the calculation the Falcon 9 first stage could to the round trip flight with orbital refueling:
The Coming SSTO's: Applications to interplanetary flight.
http://exoscientist.blogspot.com/2012/0 … ns-to.html
This was focusing on single-stage-to-orbit (SSTO) vehicles but this is not a requirement. Any currently in use medium size launcher's first stage would work. You would need low cost propellant delivered to orbit. Fully reusable launchers bringing the price to orbit down to ca. $100 per kilo would do it.
But it could also be done with the propellant obtained off-Earth. Some Mars advocates have been opposed to propellant depots since it was thought the propellant had to be obtained from the Moon, requiring giga-dollar expenditures. But it doesn't have to be from the Moon. For instance there are near Earth comets that experience outgassing, as has been observed by the Rosetta spacecraft:
Dust Whirls, Swirls and Twirls at Rosetta’s Comet. 
by BOB KING on MARCH 9, 2015
http://www.universetoday.com/119296/dus … tas-comet/
In such a case, no landing or mining would be required. You would just collect the released H2O, and CO2, CO for hydrocarbon fuel, from orbit.
Bob Clark
#513 Re: Human missions » Sponsorship for a Mars mission » 2015-06-20 14:45:38
Thanks for that. I didn't know the the advertising budgets were that high. That certainly could fund a manned Mars mission. It could also fund a robotic mission to Europa.
Bob Clark
#514 Re: Human missions » Yet another Mars architecture » 2015-06-18 07:55:23
The table would suggest that each craft would be in orbit at the same distance and speed before entering Mars to landing but that is not quite the case. We know that we can try to compansate for the differences in mass so that they do come in at the same rate of speed at aerocapture but when we see the entry results they still do not come out in the same orbiting distance. Even when we do take the time to use Aerobraking all that is for certain is that we will be lower and slower but it takes time.
I see that we have for a 21 m difference in diameter that we have a 9mt change and with just a 10 m diameter change that we have a 6mT from the 23 m diameter base line but when we use a parachute we will loss 3 mT of down mass starting point in fuel needs or are the columns starting mass switched.http://en.wikipedia.org/wiki/Aerocapture
AEROCAPTURE DEMONSTRATION AND MARS MISSION APPLICATIONS
Trajectory Guidance for Mars Robotic Precursors: Aerocapture, Entry, Descent, and Landing
A Comparative Study of Aerocapture Missions with a Mars Destination
Thanks for those links. I'm in favor of fast travel times to Mars. Rather than using propulsion to slow down, aerocapture would allow a smaller size vehicle to be used, and cheaper mission cost.
Bob Clark
#515 Re: Interplanetary transportation » VASIMR - Solar Powered? » 2015-06-05 14:37:17
Interesting report here:
Solar Electric Propulsion.
Technology Development.
www.nasa.gov/sites/default/files/files/CTaylor_SEP.pdfIt discusses the idea of using chemical propulsion for getting out of Earth's gravity well then using SEP to reach a BEO destination. Note this is how most interplanetary probes that used electric propulsion worked. The report has this surprising chart near the end:
http://oi58.tinypic.com/zt99qr.jpg
It includes the interesting fact that using the same propulsion system at 50 kW power, a 2,000 kg dry mass spacecraft would take 91 days to make the flight to Mars when running at 4,000 s Isp, but only 23 days(!) when running at 2,000 s Isp. This make sense because the thrust drops off for EP thrusters as you increase the Isp.
Hmmm. After running some numbers I wonder if that 23 day number is just the length of time it takes for the vehicle to get up to speed, i.e., it does not include the coast time.
Bob Clark
#516 Re: Interplanetary transportation » VASIMR - Solar Powered? » 2015-06-05 12:18:39
Interesting report here:
Solar Electric Propulsion.
Technology Development.
www.nasa.gov/sites/default/files/files/CTaylor_SEP.pdf
It discusses the idea of using chemical propulsion for getting out of Earth's gravity well then using SEP to reach a BEO destination. Note this is how most interplanetary probes that used electric propulsion worked. The report has this surprising chart near the end:

It includes the interesting fact that using the same propulsion system at 50 kW power, a 2,000 kg dry mass spacecraft would take 91 days to make the flight to Mars when running at 4,000 s Isp, but only 23 days(!) when running at 2,000 s Isp. This make sense because the thrust drops off for EP thrusters as you increase the Isp. But the thing is we already have a 50 kW Hall effect thruster in the NASA-457m:
Performance Test Results of the NASA-457M v2
Hall Thruster.
http://ntrs.nasa.gov/archive/nasa/casi. … 014613.pdf
I was puzzled at first by the graphic on the side listing delta-v's to reach various interplanetary destinations. For Mars, it's listed as 5.6 km/s. Since the report is discussing using EP after leaving Earth's vicinity, this must mean the delta-v just to leave Earth's position in the Solar System to Mars position, so not including the delta-v for escape velocity or slowdown delta-v at Mars.
Support for this is suggested by this:
Hohmann transfer orbit
Low-thrust transfer.
It can be shown that going from one circular orbit to another by gradually changing the radius costs a delta-v of simply the absolute value of the difference between the two speeds. Thus for the geostationary transfer orbit 7.7 − 3.07 = 4.66 km/s, the same as, in the absence of gravity, the deceleration would cost. In fact, acceleration is applied to compensate half of the deceleration due to moving outward. Therefore the acceleration due to thrust is equal to the deceleration due to the combined effect of thrust and gravity.
http://en.wikipedia.org/wiki/Hohmann_tr … t_transfer
Bob Clark
#517 Re: Interplanetary transportation » VASIMR - Solar Powered? » 2015-06-03 16:06:13
RGClark-
Please note that those numbers were given as examples and don't represent real values. The real values can be found in the following table:
https://gammafactor.files.wordpress.com … table1.jpg
I note that these nested hall thrusters are every bit as theoretical as a high power VASIMR thruster, and those numbers don't necessarily represent the mass of any engine that will ever be built.
I think GW's suggestion to use also chemical propulsion first before turning on the electric propulsion would be useful. For instance the low thrust of EP means you have to take a spiraling trajectory out of Earth's orbit. This takes more time and therefore the high speeds as shown in your table would be needed to make up for that to achieve the short travel time.
However, if you use chemical propulsion first to get out of Earth's deep gravity well then you would be just adding on the speed attained by the EP onto the velocity you would have in the Earth-Mars Hohmann transfer trajectory you attained from the chemical propulsion.
Quite likely also you would not want to depart from Earth's orbit but from a station at L2. Note that NASA just announced it wants to set a station at L2 to serve as a staging point for further BEO missions. I think the delta-v to be put on a Mars transfer trajectory in that case would only be about .9 km/s, which could easily be supplied by chemical propulsion, compared to about 3.8 km/s when departing from Earth.
You might also want to use chemical propulsion to make the trajectory closer to straight-line rather than the elliptical Hohmann trajectory. From memory I don't think this would need to be very large, about 6.5 km/s. Then the one-way delta-v that would need to be supplied by the EP would be much less than in your table.
For instance let's say chemical propulsion made the trajectory nearly straight-line and you wanted the one-way travel time to be 39 days using EP. Depending on the year, the closest Mars gets to Earth is about 60 million km. A 39 day flight is 39*24*3600 = 3,369,600 seconds. So this would require a speed of 17.8 km/s. But 6.5 km/s was already supplied by the chemical propulsion so it would actually be 11.3 km/s supplied by the EP. However, it would take some days also for the EP to build up to this speed so you would need somewhat higher speed than this to make the travel time be 39 days.
Note though this is not including slowdown time or the extra delta-v that would require. You would need then a high efficiency heat shield that would work for the high arrival speed in such a scenario. The inflatable heat shields NASA is testing now for Mars missions might work or the magnetoshell heat shields formed by magnetic fields might do it:
Magnetic bubble may give space probes a soft landing.
03 July 2014 by Robin Hague
http://www.newscientist.com/article/mg2 … nding.html
Bob Clark
#518 Re: Interplanetary transportation » VASIMR - Solar Powered? » 2015-05-24 10:04:36
In case anyone is interested, I've finally done a full analysis of the whole "39 days to Mars" thing, and shown it to be conclusively untrue. The full post is on my blog:
https://gammafactor.wordpress.com/2015/ … hang-diaz/
But this is the most important bit:I wrote:https://gammafactor.files.wordpress.com … masses.jpg
The maximum allowable mass is normalized to 1, with the mass of the engines and total system masses expressed relative to this. As you can see, they’re much, much higher.Basically, I calculated the trajectory using MATLAB, and using assumed values for various system masses I calculated how much total mass you need to make an interplanetary VASIMR cruiser happen. Turns out, you need between 12 and 40 times as much mass as it's physically possible to have and still make your mission happen.
Thanks for those calculations. As you can see from your calculated values, a major problem with the VASIMR is the mass of the engine. You cited the report:
The VASIMR Engine: Project Status and Recent Accomplishments.
AIAA 2004-0149
http://spaceflight.nasa.gov/shuttle/sup … ss2004.pdf
So with the numbers used in this report of 1 megawatt (input) power and weight to power ratio of 1 kg to 1 kW power, the engine masses in this report at ca. 1,000 kg. Then this graph indicates a thrust-to-weight ratio in the range of 1 to 1,000, or perhaps 1 to 500 if using lithium, which they say is not the preferred propellant because of difficulties of handling it.
However, already existing Hall effect thrusters can get thrust-to-weight ratios of 1 to 100:
Developmental Status of a 100-kW Class
Laboratory Nested channel Hall Thruster.
IEPC-2011-246
Table 1, Example of concentrically NHT specific mass and footprint savings, p. 5.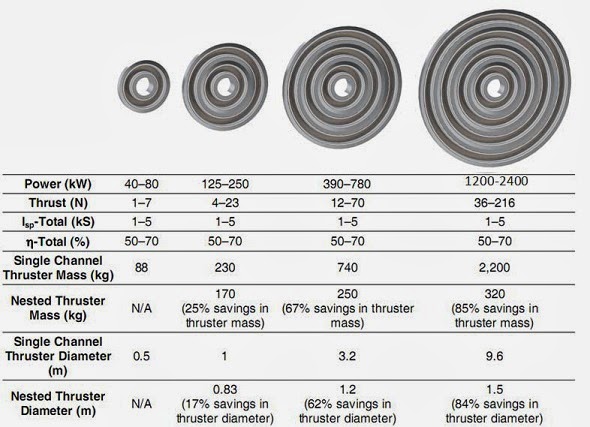
http://pepl.engin.umich.edu/pdf/IEPC-2011-246.pdf
Remember being able to get high thrust is important for this scenario of fast travel time. Otherwise it's the same scenario as with ion propulsion where the travel time would actually be slower than chemical propulsion because of the very small thrust.
Here are the numbers you are envisioning for your VASIMR vehicle:
The most important number in this chart is the Necessary Reduction Factor (NRF). It describes the ratio of necessary solid mass to the amount of allowable solid mass. For example, if you choose an exhaust velocity such that your rocket has a mass ratio of 4, and its initial mass is 80 tonnes, you can have up to 20 tonnes of solid mass. But let’s say your tanks mass 3 tonnes, your engines mass 17 tonnes, and your power source masses 20 tonnes. That would mean you would need 40 tonnes of solid mass to complete your mission, and you would have a NRF of 40/20=2. Basically, it describes how much you need to shrink down your components to make the mission feasible. For NRFs below 1, you have some amount of payload carrying capability too.
But using Hall effect thrusters you could reduce the calculated weight of 17 tonnes to 1.7 to 3.4 tonnes.
The next problem is the mass of the solar power system. Based on a 300 watt/kg specific power for the solar cells you get a 20 tonne mass for the solar power source. However, I think at least 1,000 watt/kg solar power is possible by using solar concentrators. The reason is the mirrors or lenses would be much lighter than solar cells used so you could get the same power at lighter weight.
So if the solar power sources mass, say, 7 tonnes then you see the case may indeed close for getting the total vehicle dry mass low enough to get the high speed needed, but by using existing Hall effect thrusters, not VASIMR.
Bob Clark
#519 Re: Not So Free Chat » They're watching us » 2015-05-08 05:17:23
Another indication that powers that be are reading our forum. I posted technical details about space solar panels. I included details about photovoltaic panels manufactured by Spectrolab. Today the website for Spectrolab is blocked from IP addresses outside the US. It works for an IP address inside the US. And yes, I'm a computer nerd so I know how to check that.
Thanks for that info, though I don't see why 29% compared to, say, 25% efficiency would provide that much of an advantage for say military satellites.
Bob Clark
#520 Re: Interplanetary transportation » SpaceX Falcon 9R launch » 2015-04-28 14:15:24
...
This has been true for 2-3 decades now, at the very least. If you are an engineer over about 40-45 years of age, you WILL NOT be hired by anyone! I have seen this up close and personal for over 20 years myself.
This is also why “we” seem to have lost the ability to do what our 1950’s-1960’s ancestors did. An example would be SLS: a reprise of Saturn 5 based on shuttle technology. It might or might not ever fly, but if it does, it will have taken 20+ years to do what our 1960’s ancestors did in only 6 years back then.
This is precisely because most all of engineering, not just rocketry or aerospace, is about at most 40% science written down for others to follow, at least 50% art (never written down) that was intended to be passed on one-on-one on-the-job from older mentors to the new hires, and at least 10% blind dumb luck. That’s in production work. The art and luck percentages are higher in development work.
The engineering art was never written down because managers never wanted to pay for writing it down, pure and simple. This effect was worse at contracting companies. You can tell, because only the government labs were truly “prolific” at published papers. It makes them seem smarter than they really were. The “real smarts” was in the contractors that they hired, though!
Managerial types never wanted to believe in the engineering art concept, because it meant increased spending. Hiring high-school drop-outs to do what was in the “cookbooks” was the road to increased profit, as long as you do not believe there is such a thing as engineering art. Thus there was no compelling reason to keep older engineers on the staff.
All based on a false belief system. Where have we seen that, recently? There IS recent precedent for this self-deception.
Without older guys, above about age 50-ish, there was no pass-down of engineering art to the next generation of oncoming engineers. The under-50 guys are (and were) just too busy to take this responsibility on. Since engineering art is the majority of engineering knowledge, why should it surprise anyone that none of the companies who built Saturn 5 can repeat that performance?
We have seen this play out in very recent history: Spacex had very fatal problems during the first few flights of their Falcon-1 vehicle out of Kwajalein. They did not resolve this until they temporarily hired a few old farts who actually knew the rare art of flying supersonic vehicles that stage, within the sensible atmosphere. It simply wasn’t about rocket engines, it was about aerodynamics and flight mechanics.
They have bumped into this wall again with landing Falcon-9 first stages. It’s not about the rocketry, it’s about flight mechanics, once again.
Old guys are still widely viewed as “too expensive to have on staff”. Until they consult some of us old farts, they (Spacex) will continue to crash Falcon-9 first stages. Or else they will crash many of them until they re-learn the hard way the “ancient art”. That’s my prediction.
It applies to any part of ULA as well. Including Aerojet Rocketdyne the subcontractor.
GW
Thanks for that insider info about engineering. I think I remember SpaceX presenting proudly how young the average age of their engineering staff was. Maybe. But they still need some of the "old guard" to transfer the art to the new generation.
Bob Clark
#521 Unmanned probes » Mars Express image of Phobos. » 2015-03-06 14:27:44
- RGClark
- Replies: 1
Somehow I missed this when it was released by ESA in 2010, but a great image:

http://lightsinthedark.com/2010/11/26/fear-a-flying/
Bob Clark
#522 Re: Science, Technology, and Astronomy » 7500 K Material » 2015-03-03 11:39:34
I want a material with a very high specific heat capacity. According to a quick glance at Wikipedia, Hydrogen has one of 14.3 kJ kg^-1 K^-1. Unfortunately, it's a gas...
A very high SHC would allow us to make very good heat sinks, which would enable much better thermal energy storage... and also significantly improve the prospects for stealth in space.
Perhaps H2 could be used for the fill gas for a ballute. How much total energy would need to be dissipated by a heat shield on reentry?
Bob Clark
#523 Re: Science, Technology, and Astronomy » 7500 K Material » 2015-03-02 16:05:09
I have no clues about calculating such things from first principles. I do know that zirconia makes a semi-practical material, good to about 4000 F (near 2500 K).
There are some rare-earth oxides and carbides that have been identified as "ultra-high-temperature-ceramics", or UHTC's. The best of these will go to around 7000 F (near 4000 K). These are heavy, have some structural strength in shear and compression, seem resistant to thermal shock, but conduct heat in copious quantities. They're nose tips, not insulators at all. You have a truly enormous backside heat removal problem with them.
Remember my alumino-silicate heat shield paper in Denver? I'm looking pretty closely at fibrous zirconia in porous forms now, for another combustor insulator job. The problem is structural: nobody makes a zirconia cloth as coarse as the alumino-silicate fire curtain cloth I used so long ago, which resembled boat cloth in fiberglass work. The problem is also porosity for low thermal conductivity, the antithesis of structure, unfortunately.
I am iterating toward a two-layer/two-material approach, trying to get the 4000 F temperature resistance of the zirconia next to the fire, and the structural strength of the alumino-silicate composite on its backside, where things are much cooler. What works as a low-conductivity combustor insulator might also work as a retro-radiating refractive heat shield, if surface-coated black.GW
You might want to try the NASA solicitation described in this article:
NASA SEEKING ULTRA-LIGHTWEIGHT MATERIALS TO ENABLE MISSIONS TO MARS.
OCTOBER 28TH, 2014 J.D. TAYLOR
http://www.spaceflightinsider.com/organ … ions-mars/
The due date for the Notice of Intent has been modified a few times through appendices to the solicitation. Now it's March 20, 2015, with proposals due April 17, 2015:
http://nspires.nasaprs.com/external/vie … 15ECF).pdf
The full solicitation is here:
NASA RESEARCH ANNOUNCEMENT (NRA):
NNH15ZOA001N
Space Technology Research, Development,
Demonstration, and Infusion-2015
(SpaceTech-REDDI-2015)
http://nspires.nasaprs.com/external/vie … -29-14.pdf
Bob Clark
#524 Re: Interplanetary transportation » DSCOVR Launch » 2015-03-01 00:59:28
I think I read somewhere that the barge was 370 miles downrange for the first attempt.
GW
We're you able to hear the latest full scale test of the F9 first stage:
http://www.nasaspaceflight.com/2015/02/ … ad-launch/
Bob Clark
#525 Re: Human missions » Space planes -Sierra Nevada and ESA » 2015-02-25 10:40:11
Thanks for that Spacenut. Scaled up the PRIDE vehicle might work for a manned reentry vehicle. Actually I think the IXV might work itself for a single crewman, seated. You would of course have to add life support.
Bob Clark