New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#51 2018-12-12 19:47:05
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Constant flow of the Amine solution from the bubbler tank to the heating chamber via pumps once saturated for releasal and then back through a cooling heat exchanger before send it back into the bubbler chamber to capture more co2. Pump the co2 gas from the heated chamber to be pressurized and cool as we will want to liquify it. Night time would have a cool loop to save the night cold for day time cooling use for both the co2 stage and for cooling the Amine solution for reuse.
Offline
Like button can go here
#52 2018-12-13 18:42:45
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
http://www.tcmda.com/en/Technology/Amine-technology/
http://science.sciencemag.org/content/3 … /1652.full
Amine scrubbing has been used to separate carbon dioxide (CO2) from natural gas and hydrogen since 1930. It is a robust technology and is ready to be tested and used on a larger scale for CO2 capture from coal-fired power plants. The minimum work requirement to separate CO2 from coal-fired flue gas and compress CO2 to 150 bar is 0.11 megawatt-hours per metric ton of CO2.
Offline
Like button can go here
#53 2018-12-13 21:07:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
People produce carbon dioxide while breathing and it is a the major pollutant in the premises. A typical human exhales about 1 kg of carbon dioxide per day. During sleeping in the high altitude chamber or tent one person produces(exhales) about 5,27 gallons /24 l CO2 per hour. During training in the high altitude training chambers and tents CO2 hour production rate can be 50 gallons /240 l of pure carbon dioxide(CO2) or even more. That is why CO2 level in the high altitude training facilities in most cases reaches 0,5% or even 1% CO2. ASHRAE recommends indoor CO2 levels not exceed 0,1%. During training/ exercise, the CO2 breath out from the athletic is more than 5000ppm. Without the CO2 scrubber, the CO2 level in the chamber can goes to 7000ppm in very short time. It is not health to athletic. Normal accepts level during exercise is <2000ppm.
https://www.bre.com/PDF/The-Use-of-MDEA … emoval.pdf
Example of what would be used in a room on a mars surface.
http://www.aminescrubber.com/images/AmineScrubber.pdf
Offline
Like button can go here
#54 2018-12-13 21:37:09
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,447
Re: In-Situ Propellant Production, design a opensource demonstrator
SpaceNut,
I'm not sure how these atmospheric scrubbers translate into something we can use to liquefy CO2 if the primary power consumption issue is with the pump itself. Is there a practical way to adsorb and then release enough gaseous CO2 for the LOX/LCH4 plant to operate continuously when the propellant production rate is around 1 ton per day?, Those scrubbers indicate a cycle time is 2 to 4 hours. If there is, then why don't any of the ISPP plants purport to use gaseous CO2? I think we have to bite the bullet on this one and supply enough power.
I was hoping there was a free lunch somewhere in our new technology, but there never seems to be one available.
Offline
Like button can go here
#55 2018-12-13 22:20:09
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Still searching for what will be the best options.
https://ntrs.nasa.gov/archive/nasa/casi … 016419.pdf
Compact and Lightweight Sabatier Reactor for Carbon Dioxide Reduction
https://ntrs.nasa.gov/archive/nasa/casi … 007818.pdf
Mars Atmospheric Conversion to Methane and Water: An Engineering Model of the Sabatier Reactor with Characterization of Ru/Al2O3 for Long Duration Use on Mars
Offline
Like button can go here
#56 2018-12-14 17:13:40
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
The inlet to a sabatier reactor is 5 to 7 bar or 75 psi to 100 psi for the CO2 going into it and it should be warm so as to allow the chamber temperature to be in the 200 to 400 range for best conversion. So we are as you put it need to solve for how to get what we need at less energy.
For a mars day we will have 9 hours of daylight and 16 hours of night temperature to make use of give or take and we can use concentrators for the day to elevate the temperatures on the chambers being used for co2 compession and pressurization. Sure its a slow cycle but its free energy.
Offline
Like button can go here
#57 2018-12-15 09:25:00
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Methane NIST Standard Reference Data gov. website
Re "compressing Mars air":
I'm not saying this cannot be done. I'm saying it will be difficult, and will require processes we have far less experience with.
On Earth: shop air pressures are 6 to 10 bar. The source air is at 1 bar (sea level) or 0.7 bar (10,000 feet elevation). The required pressure ratio of no more than about 10 is within the capability of an easily-built machine using either centrifugal or positive-displacement compression devices. This machine will have a fairly-high energy efficiency exceeding 50%, and the energy required to compress a unit mass of final product will be fairly reasonable.
For higher pressure ratios, only positive-displacement compression devices are feasible, and only in multistage devices. The higher the ratio, the lower the efficiency, and the higher the energy required per unit mass of final product. In the limit, this is the vacuum pump (which compresses from "nothing" to 1 bar), which has vanishingly-small efficiency and a really, really high energy to produce a tiny mass of product.
On Mars: useful "shop air" pressures still fall in the 6-10 bar range. The source is 0.006 bar range. So the compression ratio is at or higher than 1000. This looks more like a vacuum pump. Expect the same abysmal performance as a vacuum pump.
What that means is you must do something very different. The leading proposal is to cool the CO2 at night enough to freeze, mechanically harvest the dry ice frost, stuff it in a pressure-tight bucket, and warm the bucket to sublime the ice inside. If you do this "right", you might end up with a bucket of CO2 gas in the vicinity of 1 bar. That as a source could be conventionally compressed to 6-10 bar fairly readily, with efficiency and a favorable energy per unit mass of product.
What might the efficiency and energy/unit product be for the sublimation bucket? I dunno. But it is unlikely to be anything anywhere near what we experience with conventional compression here on Earth. This will be the limiting step of the process. And it will be inherently batch production, and restricted to small batch sizes, too. Batch production, especially in small batch sizes, is inherently less efficient and more energy-consumptive than steady-state stuff.
Yes, it can be done. But you-all need to stay realistic about the energy requirement and equipment masses that will be required. They will be multiple orders of magnitude larger than any "normal" compression process you are familiar with here on Earth.
Those same 1000+ compression ratios are why I personally tend to dismiss the notions of airbreathing turbine or IC engines on Mars, even if somebody dreams up a non-slagging fuel that will burn with CO2. 6 mbar "air" has more in common with the vacuum of space than any sort of Earthly air, even that atop Mount Everest. 6 mbar is the atmospheric pressure at 110,000 feet here on Earth.
GW
GW, one significant difference is that air (on Earth) is practically an ideal gas as it is far above its critical point. However, CO2 on Mars, especially if harvested during Martian night, is far beneath its critical temperature of 31C and sits close to its triple point. This means far less compressor work is needed, because the gas won't get appreciably hot under compression. Compress it to 5.1 bar and it will condense into liquid. However, frictional pumping losses would be far greater in a device compressing highly diffuse gas. The situation is very dissimilar to the compression of normal air at temperatures that we are accustomed to.
Offline
Like button can go here
#58 2018-12-15 19:51:13
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Liquid Methane transfer ISS experiment
Well, I made a presentation at a Mars Society convention about harvesting nitrogen and argon from Mars atmosphere. The purpose is not propulsion, the purpose is diluent gas for constructed Mars habitats (gas to dilute oxygen), nitrogen fertilizer for greenhouses, and argon to fill the gap between window panes for thermal insulation. Yes, you have to compress from 0.007 bar Mars ambient to 10 bar in the production canister. If this is run at Gale Crater where Curiosity is located, or the Elysium Planetia, then Mars ambient will be 0.008 bar. That isn't much better. Yes, compression will require a positive-displacement pump. Yes, compression of more than 1,000 times will require a multi-stage pump. Yes, it will have to be based on a laboratory vacuum pump. Yes, it will be inefficient and require a lot of electric power. I've said that all along. But you just have to do it. Without diluent gas, you can't produce safe breathing air on Mars. Without nitrogen fertilizer, crops won't grow. You just have to do it. So to quote the Nike slogan: "Just Do It!"
Furthermore, producing diluent gas has another problem. Start with the assumption that you just take Mars atmosphere, filter out dust and compress, freeze out CO2. The result has a major problem: carbon monoxide (CO) at lethal concentration. So you have to do something. After 9/11, NASA developed a catalyst that can be installed in a breathing mask to allow people to evacuate a burning building. The catalyst combines CO with O2 to form CO2. You still breathe CO2, but humans can withstand a lot more of that. And you can exhale CO2 out of your body; if you breathe CO, that binds to hemoglobin in blood instead of oxygen, and never lets go. That hemoglobin becomes unable to transport oxygen, and there's no way to fix it. Red corpuscles in your blood only last 100 to 120 days before they are broken down, recycled. Bone marrow makes new ones. The only way to get CO out of your body is to wait for the red blood cells (corpuscles) to be replaced. My idea for Mars is to use the same catalyst in the diluent gas production canister. Mars atmosphere has more O2 than CO, and one molecule of O2 can bind with 2 molecules of CO, so Mars has more than twice as much oxygen as required to break down all CO. The catalyst must be heated to +24°C, but the canister uses freezer coils at -100°C to remove CO2. Doing this in the same canister means CO2 produced will be removed along with CO2 from Mars atmosphere, but it means the top of the canister has a small catalyst actively heated to +24°C while the bottom has freezer coils at -100°C. That will take a lot of power!
Again, this will take a lot of power. No getting away from it, just do it.
Offline
Like button can go here
#59 2018-12-16 19:00:50
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Scientists identify new minerals for carbon capture
The minerals, members of the hydrotalcite group, are the first outside of the carbonate family to naturally capture atmospheric CO2 in mine waste, important as society continues to forge ways to lower our carbon emissions and combat climate change.
"This research confirmed that hydrotalcites are capable of sequestering atmospheric CO2 in mine waste," said Connor Turvey, who conducted this research during his PhD studies under the supervision of Sasha Wilson. "Hydrotalcites are trapping the CO2 deeper into the tailings where carbonate minerals were unable to form."
Low-cost catalyst boosts hydrogen production from water
"Our new catalyst is made from copper, nickel and chromium, which are all more abundant and less costly than platinum," says Cao-Thang Dinh, a co-lead author on the paper along with his fellow postdoctoral researchers Pelayo Garcia De Arquer and Ankit Jain. "But what's most exciting is that it performs well under pH-neutral conditions, which opens up a number of possibilities."
Seawater is the most abundant source of water on earth, Dinh points out. But using seawater with traditional catalysts under acidic conditions would require the salt to be removed first, an energy-intensive process. Operating at neutral pH avoids the high cost of desalination.
Offline
Like button can go here
#60 2019-06-29 20:42:55
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Was reminded that we could use Nitroxide and Propane for use as fuels in the technology topic for what we need.
Did a bit of searching and found that propane has a bit more kick for mass and does not need to be cryro genic for store and use just under high pressure. The reference article also indicated that the same was true for the oxidizer for use.
Abstract for Mars Sample Return Using In-Situ Propellant Production
Development of a Two-Stage Mars Ascent Vehicle Using In-Situ Propellant Production
Propane in particular offers comparable performance to methane without requiring cryogenic storage. The total MAV mass would be 119.9 kg to carry an 11 kg payload to orbit.
Mars temperature averages at around -55 deg C (218 K) with diurnal temperature swings of 100 K.
That said mining is another important element for man to stay.
Mars ISRU: State-of-the-Artand System Level Considerations
Marco polo is meantioned in document...
Offline
Like button can go here
#61 2019-06-29 22:04:33
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Getting the oxidizer is as difficult if its too energy needing.
Plasma Extraction of Oxygen from Martian Atmosphere
Making it possible with a micowave plasma chamber...
https://blink.ucsd.edu/safety/research- … toxic.html
using boiloff to pressurize the tanks
http://www.holderaerospace.com/download … erview.pdf
Offline
Like button can go here
#62 2019-10-13 21:13:23
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Found the ATK baseline here
http://fiso.spiritastro.net/telecon16-1 … 2-1-17.pdf
pg 17 for the 10kwh size reactor
Solar version:
• 4X 5.6m Ultraflex arrays
• Daytime ISRU only (1098 days to produce 4400 kg LOX*)
• Requires 4X 7.5m arrays and 1100 kg Li batteries for day/night ISRU ops excluding dust storm (527 days to produce 4400 kg LOX*)
https://ntrs.nasa.gov/archive/nasa/casi … 000235.pdf
Design of photovaltaic power system for precursor mission for human exploration of mars
Offline
Like button can go here
#63 2019-10-14 06:35:01
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,968
Re: In-Situ Propellant Production, design a opensource demonstrator
For SpaceNut re #62
Thanks for the link to the NASA paper. ATK's work is cited frequently.
The paper is encouraging but cautious... While much of the presumed technology has been demonstrated, much has not, and (it appears) a great deal of work needs to be done before a propellant manufacturing system is ready for deployment to Mars.
https://ntrs.nasa.gov/archive/nasa/casi … 000235.pdf
(th)
Offline
Like button can go here
#64 2019-10-14 08:51:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
The real work has to do with the power and mass reductions needed to have the ability to get it to mars since we can not land currently with retro propulsion to the surface from orbit only the final mile after parachuting. This is also mass limited via diameter of the heat shield to creat lift or drag to slow the ship in a very thin atmosphere.
http://fiso.spiritastro.net/telecon/Pap … 6-7-17.pdf
The planet comparison property table gives a density of approximately 1/60th of earths for a cubic meter.
The key to a solar use is not a large array but multiple arrays with many batteries at each of the nodes to aid in the creation of a grid of power such as we do on earth so as to be able to give power into the grid to compensate for those not creating as much as the others.
page 5 is where you find why the atk would be the better option for mars as its got the built in ability to width stand the mars winds, can clean the dust off ect...no extra robots and framing on the ground...
pg 25 shows the typical mars level landing field.. ouch not to level for large anything without the support foot print to keep from topling.
notice on page 26 the following of dust levels to the oposite of increasing solar energy tracking....
pg29 shows the dust is low to the ground for its density and fades as it rises above the ground.
Offline
Like button can go here
#65 2019-10-14 09:42:37
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
The mars ispp lander from nasa has been posted here in the past just not sure of the topic of MIP.
The MIP consists of a 3x3 meter, octagonal lander which will house an atmosphere processing module, a fuel liquefaction module, a water processing module, a water cleanup module, and a soil processing module. Labscale - demonstration MIP lander components have been developed and constructed as a NASA cross-center effort.
https://www.nasa.gov/pdf/203084main_ISR … 7%20V3.pdf
NASA Lunar In-Situ Resource Utilization (ISRU) Development & Incorporation Plans
https://ntrs.nasa.gov/archive/nasa/casi … 004402.pdf
Human Mars Lander Design for NASA’s Evolvable Mars Campaign
https://ntrs.nasa.gov/archive/nasa/casi … 033701.pdf
https://www.lpi.usra.edu/meetings/isru97/pdf/2401.pdf
https://ntrs.nasa.gov/archive/nasa/casi … 005871.pdf
Offline
Like button can go here
#66 2019-10-14 13:27:30
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
The purpose of In-Situ Resource Utilization (ISRU), or “living off the land”, is to harness and utilize space resources to create products and services which enable and significantly reduce the mass, cost, and risk of near-term and long-term space exploration. Which provide the means to support and stay as part of mans long term goals of space exploration.
https://space.nss.org/media/2005-ISRU-roadmap.pdf
NASA In-Situ Resource Utilization (ISRU) Capability Roadmap Final Report May 19, 2005
http://www.marsjournal.org/contents/200 … ke2005.pdf
NASA In-Situ Resource Utilization (ISRU) Capability Roadmap Executive Summary
https://www.nasa.gov/sites/default/file … s_isru.pdf
In-Situ Resource Utilization (ISRU) Capabilities
For every 1 kg landed on Mars, 7.5 to 11 kg has to be launched into orbit from Earth.
23 mT of oxygen and 6.5 mT of methane propellants are needed for the Mars crewed ascent vehicle. This equates to the payload mass of 3 to 5 SLS launches
Which is why we will not be bringing stuff to mars that makes it so we cannot stay. Sure we can return short stay but thats not what we want.
Potential Lunar Resource Product Needs
‒2,000 kg oxygen (O2) per year for life support backup (crew of 4)
‒3,500 kg of O2per lunar ascent module launch from surface to low lunar orbit
‒16,000 kg of O2per reusable lunar lander ascent/descent vehicle to L1/L2 (fuel from Earth)*
‒30,000 kg of O2/Hydrogen (H2) per reusable lunar lander to L1/L2 (no Earth fuel needed)*Potential Mars Resource Product Needs
‒20,000 kg to 25,000 kg of oxygen (O2) per ascent mission (~2 kg/hr)
‒5700 kg to 7150 kg of methane (CH4) per ascent mission
‒14,200 kg of water (H2O) per ascent mission
https://rascal.nianet.org/wp-content/up … zation.pdf
Mobile In-Situ Water Extractor (MISWE) for Mars, Moon, and Asteroids In Situ Resource Utilization
Offline
Like button can go here
#67 2019-10-14 16:29:24
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Sorry all for the data mining of documents for information for the subjects of Mars for Man.
https://sciences.ucf.edu/class/wp-conte … chesV1.pdf
Human Mission Architectures and Approaches and Concepts for IncorporatingIn Situ Resource Utilization (ISRU)
Every 1 kg of propellant made on the Moon or Mars saves 7.4 to 11.3 kg in LEO
For large payloads to mars means not using parachutes to go with when we need retro propulsion to land with.
14.2 MT of Mars water per mission requires excavation of a Football field to a depth of 1.1 to 9.6 cm! (30% to 3% water by mass) Excavation rates required for 14.2 MT H2O/mission production range based on water content
– Hydrated soil (3%): 41 kg/hr
– Icy soil (30%): 4 kg/hr
pg 18 gives and idea for what can be done as we process the consumed aspects of a mars mission
pg 20 is The Chemistry of Mars ISRU and steps for making what we need
pg 42 gives the processing and from what source to get the much needed water, oxygen
Mars sustainability, backup and mobility future via fuel cell use
Life Support Backup (DRM 3)
4500 kg of O2
3900 kg of N2
23,200 kg of water (H2O)Mobile Power
Fuel cell and reactant storage
Amount: 1000 kg O2& 350 kg CH4per 14 day traverseHabitat Backup Power
Fuel Cell reactants for Dust Storms
14.8 KW at up to 120 days
Amount: 21 mT O2& 9 mT CH4
https://www.aaai.org/Papers/Symposia/Sp … 04-002.pdf
Model Checking Autonomy Models for a Martian Propellant Production Plant
www.sei.aero/archive/SEI_ISRU_Market_ISDC_25May2007_vA.pdf
ECONOMIC ANALYSIS OF LUNAR PROPELLANT PRODUCTION:
https://www.lpi.usra.edu/meetings/marsc … f/4208.pdf
EARLY IN-SITU RESOURCE UTILIZATION (ISRU) LEADING TO ROBUST SAMPLE RETURN AND HUMAN EXPLORATION MISSIONS.
Offline
Like button can go here
#68 2019-10-14 17:39:59
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
Location
Moon
Product Amount (kg)Need/Time Use
O2 1000 Per Year Crew Breathing - Life Support Consumable Make up
O 23000 - 35002x Per Year Non-Reusable Crew Ascent Vehicle Propulsion - Surface to Low Lunar Orbit: Earth fuel
O2~160002x Per Year Reusable Ascent/Descent Propulsion - Surface to L1/L2: Earth Fuel (4000 kg payload)
O2/H2~30,0002x Per Year Reusable Ascent/Descent Propulsion - Surface to L1/L2 (4000 kg payload)
H2O 150,0002x Per Year Lunar Human Outpost & Reusable Transportation
O2/H2 150,000 Per Year Anount needed for Propellant Delivery to LDRO for Human Mars MissionMars
O2/CH4 22,728/6978 Per Use/1x 480 Days Non-Reusable Crew Ascent Vehicle Propulsion - Surface to High Mars Orbit
O2/CH4 59,000/17,100 Per Use/1 or2x Per Yr Reusable Ascent/Descent Propulsion - Surface to Mars Orbit
H2O 3,075Surface/500 DaysLife Support System Closure
H2O 15,700 Per Use/1x 480 Days Extracted
H2O to Make Non-Reusable Ascent Vehicle Propellant
H2O 38,300 Per Use/1 or2x Per Yr Extracted
H2O to Make Reusable Ascent/Descent Vehicle Propellant
Offline
Like button can go here
#69 2019-10-14 18:50:44
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
An important part of any system that takes in a very dusty mars air at times....
https://ntrs.nasa.gov/archive/nasa/casi … 005058.pdf
Martian Atmospheric Dust Mitigation for ISRU Intakes via Electrostatic Precipitation
https://www.nasa.gov/sites/default/file … -24-16.pdf
Mars Water In-Situ Resource Utilization (ISRU) Planning (M-WIP) Study
I see this one has 4x kilowatt power plants in one of the pages....
Of course this what we need to even get fuel creation from the atmosphere and that is because we have landed and are ready to go home in time.
https://ntrs.nasa.gov/archive/nasa/casi … 016019.pdf
Mars Ascent Vehicle Design Considerations
MARCO POLO is a NASA ISPP experiment to produce LOX/LCH4 using Martian regolith and atmosphere.
http://kiss.caltech.edu/workshops/isru/ … anders.pdf
Mars ISRU: State-of-the-Art and System Level Considerations
MOXIE is just a SOXE and O2 liquefaction experiment.
MARCO POLO is a complete ISPP plant.
https://mepag.jpl.nasa.gov/meeting/2011 … -06-16.pdf
https://ntrs.nasa.gov/archive/nasa/casi … 023504.pdf
https://wordlesstech.com/marco-polo-mars-pathfinder/
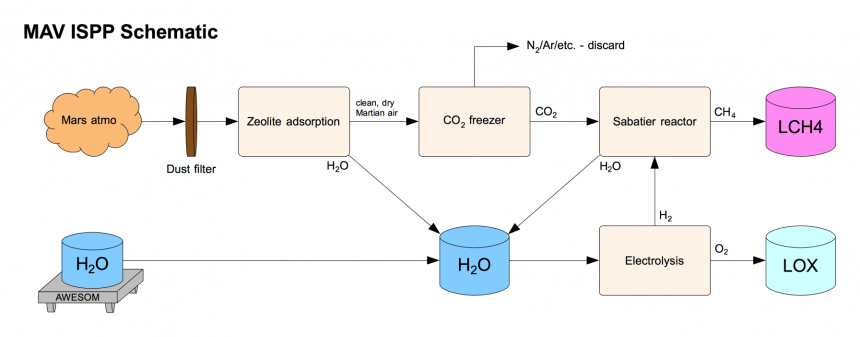
https://ntrs.nasa.gov/archive/nasa/casi … 008610.pdf
https://ntrs.nasa.gov/archive/nasa/casi … 001775.pdf
New topic created by louis based on link post
What is required in terms of mass to produce enough propellant on Mars for a return Starship?
This is an interesting analysis from Blake on Reddit:
https://www.reddit.com/r/spacex/comment … ant_plant/
He comes up with a combined figure of only 74 tons for both a 1 Mwe Propellant Production Facility and the mass of the PV facility generating the power for the process.
Offline
Like button can go here
#70 2019-10-16 22:43:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
http://www.niac.usra.edu/files/studies/ … ngland.pdf
Mars Atmosphere Resource Recovery System
https://www.stirlingcryogenics.eu/files … cation.pdf
Stirling Cryogenics liquid air plant
Offline
Like button can go here
#71 2019-10-22 20:55:13
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
An ISRU Propellant Production System to Fully Fuel a Mars Ascent Vehicle
https://www.aiche.org/conferences/aiche … ic-methane
https://www.researchgate.net/figure/Cro … _318511857

Enhanced CO2 Methanation by New Microstructured Reactor Concept and Design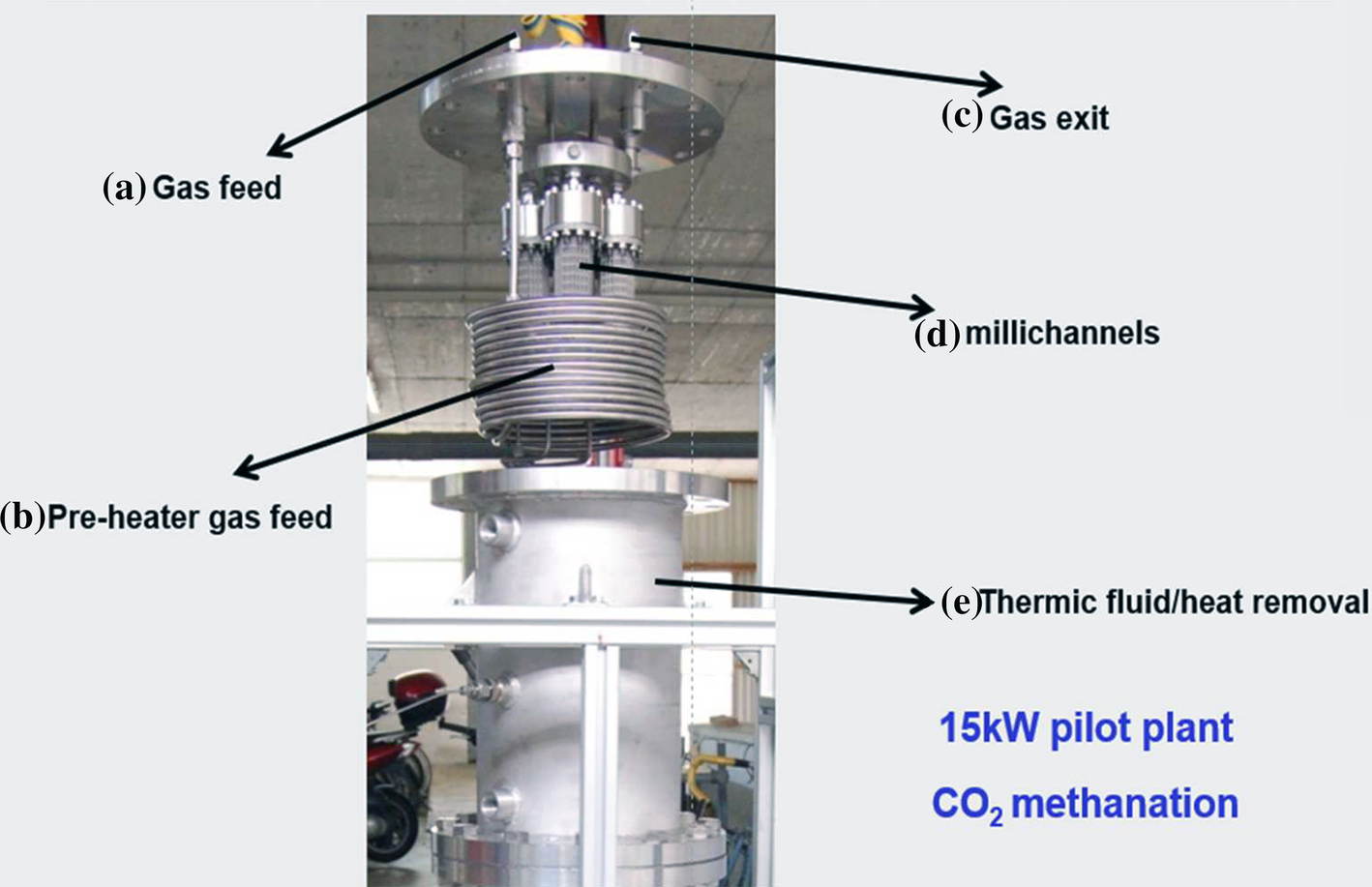
http://chateauhetsakais.com/filtering-reverse-osmosis
Thermodynamic modelling of the methanation process with affinity constraints

Some have flow valves, check valves and shutoffs in inputs and outputs including the cooling loops as well as the heating sections.
Offline
Like button can go here
#72 2019-10-23 03:38:13
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: In-Situ Propellant Production, design a opensource demonstrator
I note that the Awesom Co appears to have a pallet depot on Mars. Would that be a subsidiary of the ACME Corporation?
Offline
Like button can go here
#73 2019-10-23 04:08:17
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,968
Re: In-Situ Propellant Production, design a opensource demonstrator
For elderflower re #72
Thanks for the trip down memory lane!
https://en.wikipedia.org/wiki/Acme_Corporation
(th)
Offline
Like button can go here
#74 2019-10-23 04:09:41
- elderflower
- Member
- Registered: 2016-06-19
- Posts: 1,262
Re: In-Situ Propellant Production, design a opensource demonstrator
Look out for coyote shaped impact craters!
Offline
Like button can go here
#75 2019-10-23 19:45:02
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: In-Situ Propellant Production, design a opensource demonstrator
https://learn.openenergymonitor.org/sus … er-process
We have another method to make methane with our waste stream as well Make a Biogas Generator to Produce Your Own Natural Gas
Sure we will not have many grass clippings but once we are gardening we will have waste from the no so edible parts of the pants to make use of, food waste as I am sure left overs will not be as good and its going to be a long time to livestock manure for a renewable source of biogas energy with a homemade biogas generator.
Offline
Like button can go here