New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#51 2023-05-31 09:27:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
Earlier in this topic I posted a link to a marine (model) steam engine that was on offer for $99 USD
Today I asked Google to look again, and it found several vendors ... the one at the link below apparently serves the model boat builder community, and it has some serious engines for the serious builder. At the low end if offers one for $160 USD.
https://www.enginediy.com/products/micr … v_EALw_wcB
Here is a link to the site: https://www.enginediy.com/products/
In reading comments from customers, I ran across mention of using air as a way of testing a steam engine. I have seen this method before at trade shows, where machinery is put into motion without endangering the public.
This led me to consider an option to consider for a model/demo system ....
If liquid air is held in a tank, then it might be brought to a gaseous state by passing the liquid through a Thermal Energy Store. The resulting gas might be of sufficient pressure to operate a model steam engine, without the fuss and bother of generating actual steam.
This is a variation of an idea proposed earlier in this topic, to avoid dumping energy to air by carrying a cold sink as part of a package.
In this case, the energy supply would be held in a combination of the cold air and the hot energy store, whatever it's nature might be.
The air could then be exhausted to the atmosphere after performing work in the engine.
(th)
Offline
Like button can go here
#52 2023-05-31 09:50:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
In the transcript below, I invited ChatGPT to consider a situation with air flow instead of steam. It still seems to be hung up over the features of the specific piston engine to be used. I think such details should not matter, because we have the power needed at the input of the engine (416 watts for an hour).
Never-the-less, I'll attempt to find the specifications for the $160 engine.
https://docs.google.com/document/d/1dyF … sp=sharing
(th)
Offline
Like button can go here
#53 2023-05-31 10:02:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
As a follow up, here is a contact form request just submitted to the manufacturer of the $160 USD model steam engine.
Newmars.com/forums is a division of Mars Society (marssociety.org)
A team is investigating thermal energy storage. A demonstration system might include a small piston engine, such as your $159 two cylinder model. ChatGPT seems to need specifications of your engine in order to know what pressure must be supplied at the input port, to drive the engine at a rate that will consume 416 watts at the input.
You can see the discussion on public display in:
https://newmars.com/forums/viewtopic.php?id=10510Do you have a publicly available document with the specifications ChatGPT is requesting? Or can you simply provide the pressure needed to deliver 100 watts at the load. We are assuming 30% efficiency for the piston engine, and 80% efficiency for the generator.
Thanks!
tahanson43206
(Junior) Moderator
NewMars.com/forums
(th)
Offline
Like button can go here
#54 2023-05-31 18:00:02
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
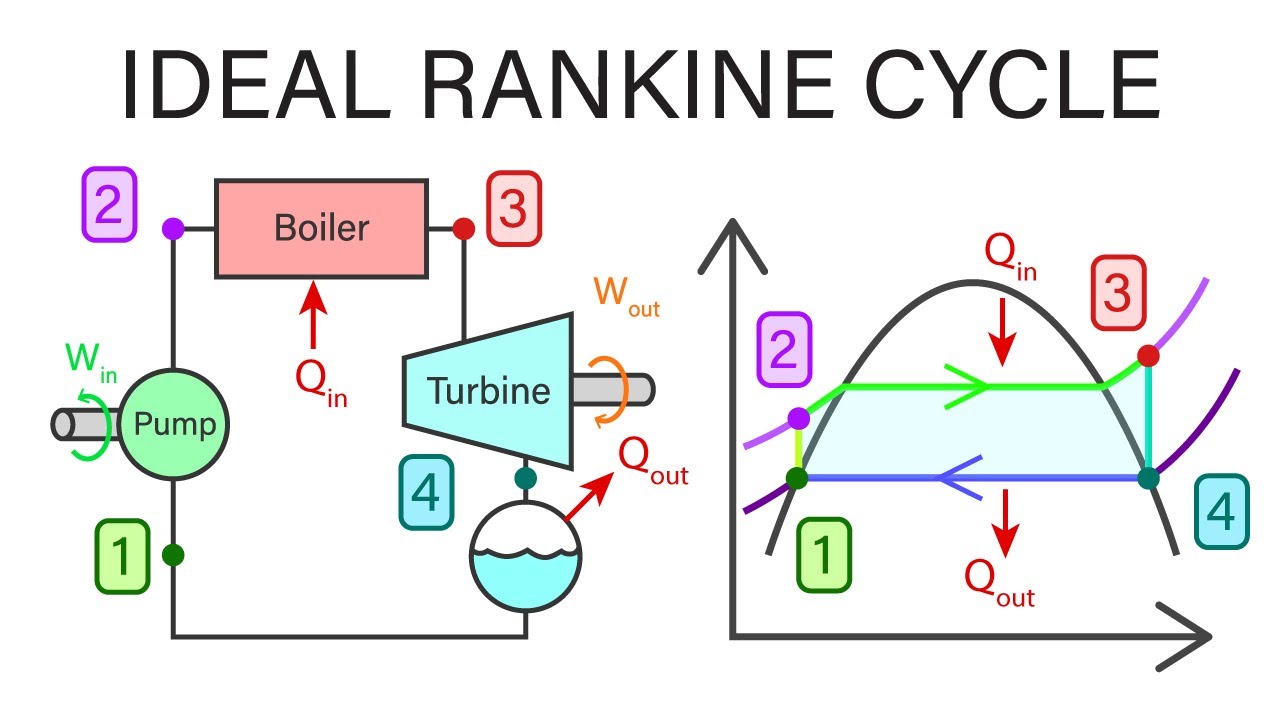
Something read today about the higher-pressure side is that after heating to entering the turbine we will have 30 Bars for freon like working fluids at point #3 and after the cooling it will drop down to 2 Bar at point labeled #1 and that will mean the pump is required to push it into the heating section. That power is going to need a different source from the one created since we are going to need to inject pressure on startup for heating cycle after being bypassed for the night or while stopped for long periods of time.
I believe the design called for 30kw of heat for hot water and just 5 kw of electrical since the cooling radiators were electric fans.
Another factoid is that a gas engine of 1.4 liter size getting roughly 32% efficiency will be dumping 45 kw out of the cooling radiator and out the exhaust we are losing another 120kws. Knowing the sum is 165kw and the 32% efficiency we now know the engine is providing 78kw for wheel use out of the total 243kw from the energy source.
This is what we need from our heat battery if we are having car like performance for each hour of driving at 60 mph.
Offline
Like button can go here
#55 2023-06-01 17:23:07
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
Apologies for not posting more sooner. I have been on leave in a remote part of Scotland. Internet access has been intermittent and the holiday has been busier than I thought it would be.
I think a steam cycle is a workable option for a large land based or ship based powerplant, using stored heat. The problem with doing this with something as small as a car is that the steam must either be superheated or dried before putting it into a turbine. Superheat requires temperatures greater than 374°C (H2O critical point). Drying steam that is not superheated requires bulky swirl-vane steam dryers. A car will be too space constrained for steam dryers. A third option is to either limit the turbine to low rotational speeds, allowing it to tolerate wet steam, or use a piston steam engine, with drains on each cylinder. Both options are possible, but end up being bulky for the amount of power produced. On a ship or land based powerplant, this is less problematic, because we have greater scale to work with. But I cannot see this working with something as small as a car.
The problem with a homemade S-CO2 engine is high pressure. The studies I have seen talk about pressures between 70 and 250bar. That is a lot of pressure and it makes the device into a potential bomb. Pipework needs to be seemless boiler tube. In most industries using pressures that high, welds are tested using cobalt-60 sources to identify any discontinuities in the welds that could fracture under pressure. Even a small diameter weld that fails at 200bar, can produce fragments that move at the speed of a pistol bullet. I could design the power generation system. But we need to think about safety precautions.
Last edited by Calliban (2023-06-01 17:44:11)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#56 2023-06-01 19:52:01
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,447
Re: Limestone-based Thermal Battery
tahanson43206,
You might have a point about using water instead of these specialty materials. The quantity of A36 steel I can afford to buy is well in excess of the amount of stainless I can afford to buy, it's very easy to weld, and I have the most experience welding this type of steel. A36 is very inexpensive, it's easy to obtain because everybody makes it, and I know where I can get as much scrap as I want. It's not as strong as more expensive steels, but using enough of it gets the job done. The vehicle becomes, but the amount of power we can extract is still sufficient for 100 miles of driving range. That's what I'm after since 100 miles covers 95% of real world driving.
Anyway, I intended to circulate a refrigerant gas through the hot water tank to absorb heat via an automotive AC radiator, cool it back down using another automotive AC radiator, and then run the refrigerant gas through a Stirling piston pump motor. That's perfectly doable using scrap sheet steel, junk yard truck parts, and whatever I can purchase from hardware or automotive stores.
Maybe if Calliban ever gets back to us, we can reconsider using very hot limestone and CO2, but a hot water battery is inordinately easier to pull off. It's a giant water heater tank, a pair of automotive AC system radiators, and an air pump. This is something I can figure out myself without advanced engineering knowledge.
Offline
Like button can go here
#57 2023-06-01 20:18:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
For kbd512 re #56
Thanks for your development of the Thermal Battery idea in a form that would allow measurement of performance in the Real Universe!
The first version of a system will (at least usually) provide a starting point for improvements, while doing the critical work of proving the underlying theory.
Best wishes for success at each stage of the project!
It is good to see Calliban's report on what sounds like a successful (ie, busy) vacation away from the office!
(th)
Offline
Like button can go here
#58 2023-06-04 16:33:26
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
Somewhere between the compressing pump and connection labeled 2 is a set of switches to control whether the pump is active or not needed as we have enough to make it into the chamber. That cut in is low pressure and cutout for being too hi so that we stop the unit.
Things that we do not know is the size of the tubing that we will be making use of and the section lengths required for each change in pressure level. We also need the bypass length as well to solve the closed off loop pressure static state.
Those satisfy the volume number for the equation to know the values of pressure and such for the loop while running.
Offline
Like button can go here
#59 2023-06-04 19:26:35
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
For kbd512 and Calliban re Thermal Energy Storage battery ....
After some back-and-forth, it appears that SpaceNut and I have settled on (or at least appear to have settled on) a power intake we'd like to see from your system.
To achieve a demonstration of 100 watts of power at the final destination (a set of light bulbs) it appears your design needs to be able to provide 7 cfm at 90 psi for an hour.
We could probably improve the efficiency, but the delivery expectation appears to be reasonable.
Please provide the design for a TEB that can provide that output for an hour.
To avoid over complicating things at this early stage, please assume a stationary installation.
(th)
Offline
Like button can go here
#60 2023-06-05 16:37:08
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
A steam based car is easier than S-CO2 to construct as a home project. The practicality of S-CO2 depends upon the engineering of compact, printed heat exchangers. Water is denser, with better thermal conductivity and higher volumetric heat capacity than CO2, even if the latter is compressed to 20MPa. And boiler and condenser tubes can be thin walled, as the pressures involved will be lower with a steam cycle. So heat exchangers will be more compact with a steam cycle, even if the turbine is not.
The water input to the steam cycle could be preheated to 100°C and stored in a seperate insulated tank within the vehicle. Why would we do that? Water has a very high specific heat. A single kg of water heated from 20 to 100°C, will store as much energy as 1kg of limestone heated from 20 to 300°C. This is important because water can be easily heated to 100°C using concentrated solar heat or CHP plants. By preheating the water entering the limestone thermal store heat exchanger tubes, the amount of expensive electricity needed per km of travel, can be reduced substantially.
In some climates, there is enough sunshine available year round, to raise temperatures of 300-400°C using parabolic tough collectors. A stationary hot rock thermal store could store this heat and transfer it to vehicles using pipes containing hot oil. Small modular pressurised water reactors could provide direct heat to thermal charging stations in cold climates. Whilst our demonstration vehicle will be electrically charged, we could eventually develop the concept to recharge the vehicle using direct heat, thereby avoiding electricity altogether.
For a steam car using a compact turbine, steam must be dried. I am assuming that we can build a compact enough steam dryer to do that job. The boiler itself will consist of pressure tubes running through the thermal store. These will exit into a water-steam seperator, which is essentially a tank, with the steam dryer built into the tank. The seperator / dryer will need to be a pressure vessel. I don't think there is anyway of escaping that requirement, unless we can reliably produce temperatures in the superheat range even at the lowest operating temperature of the limestone store.
I have a rather ancient book on steam engines somewhere in my collection. It is so old that most of the formulae it contains are imperial. I will dig it out at some point this week. Hopefully it can provide some information on steam drying. For powerplant turbines, the HP and MP parts of the turbine are both located in the same pressure shell. If the steam entering the HP is superheated, then there isn't usually any need for intermediate drying or reheat between the HP and MP turbines. But the pressure and temperature drop between the MP and LP can produce condensation that will destroy the LP blades if the steam is not dried. To minimiee the need for additional steam drying and drains between the two stages, I propose that we reheat the steam by passing it through the thermal store again. This reheat stage will reduce the moisture content of the steam, hopefully negating the need for an LP dryer.
You asked previously if carbon steels can be used instead of stainless. The answer is yes. But keeping corrosion under control in a carbon steel system requires that the boiler water is maintained at a pH of about 11. Back in the good old, bad old days, boiler ash woukd be added to steam engine boilers to keep the pH alkali. Nowadays, we woukd probably use lime (calcium hydroxide) to achieve the same result. I would avoud caustic soda, as sodium ions tend to accelerate steel corrosion.
Going off subject a little, any stored heat engine that can work for a car, will presumably work even better for larger vehicles like trains, buses and ships. The limestone heat store also has applications for grid electricity storage. At 1100°C, the CO2 equilibrium vapour pressure over calcium carbonate is about 9 bar(a), or 8 bar(g).
https://en.m.wikipedia.org/wiki/Calcium_carbonate
The specific heat of limestone roughly double between room temperature and 1000°C.
https://www.researchgate.net/figure/Var … _325162542
This means that each kg limestone will store 2x as much heat between 900-1100°C, as it does between 0-200°C.
If intermittent electricity is used to charge a limestone thermal store in a steel or concrete pressure vessel, then we can use the CO2 cover gas to transfer heat into boilers. If steam temperatures as high as 700°C can be achieved, then electricity can be generated with efficiency up to 50%. But even a 500°C steam temperature can achieve a 40% recovery efficiency. The waste heat from the cycle could be used for district heating, especially if the thermal stores are constructed outside of towns and cities. So overal exergy efficiency at 500°C could be ~60%, rising to 66.7% at 700°C.
Last edited by Calliban (2023-06-05 17:14:59)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#61 2023-06-05 17:32:43
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
Today I watched how fast the coolant temperature would rise in the Subaru which is 2.5 l engine and its less than 10 minutes as compared to a gas engine of 1.4 liter size dumping 45 kw out of the cooling radiator would means that my vehicle is dumping almost twice as much heat.
I am saddened to not be able to turn that waste into electricity or heat storage for later use as that is enough to power the house for the day..
I also agree that using a solar concentrator in a large parking lot could recharge the limestone battery while idle but what would you do on that cloudy rainy day?
.
Offline
Like button can go here
#62 2023-06-05 18:03:49
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
Today I watched how fast the coolant temperature would rise in the Subaru which is 2.5 l engine and its less than 10 minutes as compared to a gas engine of 1.4 liter size dumping 45 kw out of the cooling radiator would means that my vehicle is dumping almost twice as much heat.
I am saddened to not be able to turn that waste into electricity or heat storage for later use as that is enough to power the house for the day..I also agree that using a solar concentrator in a large parking lot could recharge the limestone battery while idle but what would you do on that cloudy rainy day?
.
The charging stations would be located outside of towns, where there is plenty of space for the solar collectors. The collectors would not charge the cars directly, but would heat a stationary thermal store which would provide day-day or even annual heat storage. The store would be a giant stack of limestone within a steel silo, covered with dirt for insulation. The cars would charge up from the thermal store. A heat transfer fluid (mineral oil?) would transfer heat from the store to the car. If we are charging the store using a small nuclear reactor, then the store can be much smaller, because the reactor can produce heat 24/7.
Last edited by Calliban (2023-06-05 18:04:49)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#63 2023-06-06 06:35:10
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
For Calliban re parallel inquiry about Thermal Energy Battery ....
In a parallel discussion, SpaceNut and I seem to have arrived at an affordable configuration of equipment that can be set up to provide a load for a Thermal Energy Storage system of some kind.
The input required for this particular set of equipment is a feed of 7 cubic feet per minute of room temperature air, and the proposed test duration is one hour.
Please design a Thermal Energy Storage system able to deliver that volume of air.
At your discretion, please consider combining a cold store (liquid air) along with hot store you've been discussing with kbd512.
By feeding the liquid air into the hot store, you would eliminate the need to interact with the external environment, just as an electric battery is able to supply power to a vehicle without interacting with the external environment.
This would be a stationary installation for test purposes, so size of the components is unlimited, or at least much less limited than would be the case for an automotive application.
(th)
Offline
Like button can go here
#64 2023-06-06 20:42:57
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
For kbd512 and Calliban re Thermal Energy Storage system ....
ChatGPT suggested Ammonia as a working fluid to pull thermal energy out of air to make liquid air for a cold sink, and to push that thermal energy into the the hot material you will ultimately select. The advantage of designing your "battery" with both hot and cold is that you are not dependent in any way on the external environment.
I am hoping your posts might include calculations to see what such a system might look like.
(th)
Offline
Like button can go here
#65 2023-06-07 04:33:21
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
For Calliban re parallel inquiry about Thermal Energy Battery ....
In a parallel discussion, SpaceNut and I seem to have arrived at an affordable configuration of equipment that can be set up to provide a load for a Thermal Energy Storage system of some kind.
The input required for this particular set of equipment is a feed of 7 cubic feet per minute of room temperature air, and the proposed test duration is one hour.
Please design a Thermal Energy Storage system able to deliver that volume of air.
At your discretion, please consider combining a cold store (liquid air) along with hot store you've been discussing with kbd512.
By feeding the liquid air into the hot store, you would eliminate the need to interact with the external environment, just as an electric battery is able to supply power to a vehicle without interacting with the external environment.
This would be a stationary installation for test purposes, so size of the components is unlimited, or at least much less limited than would be the case for an automotive application.
(th)
That would work, but it is no longer something that Kbd512 can build in his home workshop. We are talking about high pressure gas turbines and cryogenic pipework.
A steam based system would be the easiest to build at a small scale. The problem with a steam based heat engine is low efficiency. If we are producing steam at a temperature of 400°C and condensing it at say 50°C in a small steam engine, then realistic efficiency is going to be about 25%. A steam car would consume about 3x more electrical energy than a battery electric car. Unless electricity is really cheap, it would end up being a more expensive solution overall.
There is one solution that would improve efficiency at the expense of reducing vehicle range. This would be for the car to carry both a hot rock store as the heat source and a tank of cold water as the cold source. The hot rock store would generate steam that is input to the HP turbine. The MP turbine would exhaust into a condenser tank, into which cold water is sprayed. As you conduct your drive, your heat store gets colder and the condenser tank fills up with hot water. The heat to mechanical work efficiency woukd be low - probably no better than 20%. But at the end of the drive you wouod have a tank of almost boiling water that you pump into your house and use for heating or washing. That way, the energy recovery efficiency would be ~100%. The exergy efficiency would be at most 60%, because we are converting electricity into mostly low grade heat. But 60% is a lot better than 25%. If we consider a human beings whole energy consumption, a steam car would be affordable so long as they can reuse the waste heat.
What sort of range would the car get? I keep going back to the Tesla 3, which can travel 7km foreach kWh of battery energy. Let us assume a steam car that gets 7km of range for each kWh of mechanical work. Let us further assume 20% heat to work efficiency. I am going to further assume that we heat limestone to 750°C and cease steam production when temperature drops to 250°C. To provide 5kWh of primary heat, we would need 33kg of limestone. How much water would we need to absorb 4kWh of heat? Assume water starts at 20°C and is heated to 100°C.
M = (4 x 3,600,000)/(80×4200) = 43kg.
So 33kg of limestone and 43kg of water, allow us to release 1kWh of work and capture 4kWh of waste heat. Let us assume a total energy store mass of 1 tonne. The total work energy stored by our 1 tonne of hot rock and water would be:
Q = 1000/(33+43) = 13.2kWh
If 1kWh of work energy takes us 7km, then 13.2kWh of work energy would give a range of 92.4km (57 miles). If you can be satisfied with a car that will drive you 57 miles in a day and provide your famility with hot baths and showers at the end of it, then this would be a practical solution.
Last edited by Calliban (2023-06-07 04:51:56)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#66 2023-06-07 06:32:55
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
For Calliban re 57 mile update of the Stanley Steamer
Thanks for developing the water based cold store concept for a possible transportation vehicle.
If the "refueling" station has a matching design, the hot water could be exchanged for cold while the hot rock store is recharged with electricity. At home, the garage recharging facility would take in the hot water and supply cold water and cool the water back down over night. The heat of the water might keep the garage warm in winter, which would contribute to efficiency.
The steam engine is going to need lubricants to keep running, so there would be some consumption of hydrocarbons, but it would be small compared to burning them.
I wonder (a bit) about the mass needed for the energy store, and all the related components. That would be a robust vehicle, so wheels and undercarriage would need to be build with corresponding strength and durability.
All that said, this may be a vision worth pursuing because it reduces consumption of hydrocarbons, and the renewable energy to charge the system is free after capture equipment is paid for.
Finally, thanks for giving the liquid air variation a nod. If you are so inspired, please give that alternative a similar treatment. I expect the range will increase and the mass will decrease, which would imply a mass production opportunity.
The steam engine would be replaced with an air motor, which would itself require lubrication.
From this site: http://stanleymotorcarriage.com/General … hnical.htm
I learned that the Stanley Steamer had a range of 150 to 250 miles on a 24 gallon tank of water. The kerosene tank was 20 gallons.
(th)
Offline
Like button can go here
#67 2023-06-07 19:48:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
The closing line of fuel economy is why we are looking to change how we are providing motion for a vehicle for other types of stores as fuel sucks for miles for gallons used..
Offline
Like button can go here
#68 2023-06-08 20:41:28
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
Kbd512 post of construction of box and materials
With the typical automobile having an engine compartment and a behind the back seat location for a limestone battery to reside we know that heat tubing will need to have lengths based on the overall lengths from front to back for the most part.
If the battery is sized for the back locations its overall shape so as to not take up all of the cargo space is most likely a 1ft x 1ftx 3ft side to side measurement of the vehicle with the outer case for heat isolation of possible an inch or 2 filled with insulation and a vacuum if we can achieve that.
Engine compartment is more like a 2 ft cube with the same outer shell same as the other box configuration.
With in both is the limestone, heating element if that is the preferred method of recharge and tubing for the loop power exchange system to create power from.
Drive train can be with or without transmission coupled electric motor, regenerative systems, battery to save energy between cycles.
A 1m diameter spherical lump of limestone would have surface area of 3.14m2. Lets assume a 10cm thick layer of crushed limestone in vacuum, as insulation and a 500°C temperature difference. Thermal leakage would be:
Q = KA × dT/dX = 0.001 × 3.14 × 500/0.1 = 15.7W.
A 1m sphere of limestone would weigh 1.4 tonnes. Heated to 520°C, it would store some 194kWh of heat. It would take 124 hours for the sphere to lose 1% of its heat by conduction.
Calliban post made on power requirements to move the vehicle
So, what happens if the box to hold the heat is 100 centimeters * 100 centimeters * 10 centimeters = 100,000 centimeters^3 to capture the heat from an exhaust?
What is I made use of the space a standard 11-gallon gas tank used for the size of the limestone tank?
That means we are able to have 41,639.5 cm^3 or half of the above size.
https://www.aqua-calc.com/calculate/vol … /limestone
1 cubic meter of Limestone weighs 2,711 kilograms [kg]
So, a gas tank would hold 135 kg approximate of limestone to store heat in.
What sort of range would the car get? I keep going back to the Tesla 3, which can travel 7km foreach kWh of battery energy. Let us assume a steam car that gets 7km of range for each kWh of mechanical work. Let us further assume 20% heat to work efficiency. I am going to further assume that we heat limestone to 750°C and cease steam production when temperature drops to 250°C. To provide 5kWh of primary heat, we would need 33kg of limestone. How much water would we need to absorb 4kWh of heat? Assume water starts at 20°C and is heated to 100°C.
M = (4 x 3,600,000)/(80×4200) = 43kg.
So 33kg of limestone and 43kg of water, allow us to release 1kWh of work and capture 4kWh of waste heat. Let us assume a total energy store mass of 1 tonne. The total work energy stored by our 1 tonne of hot rock and water would be:
Q = 1000/(33+43) = 13.2kWh
If 1kWh of work energy takes us 7km, then 13.2kWh of work energy would give a range of 92.4km (57 miles). If you can be satisfied with a car that will drive you 57 miles in a day and provide your famility with hot baths and showers at the end of it, then this would be a practical solution.
Offline
Like button can go here
#69 2023-06-09 18:34:49
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,976
Re: Limestone-based Thermal Battery
Mars_B4_Moon posted a link to an article about discoveries in the study of bosons. In the site Mars_B4_Moon showed us, I found an article about investigation of ways to improve insulation:
https://phys.org/news/2023-06-energy-ma … nisms.html
I thought this might be of interest to the Thermal Battery topic.
(th)
Offline
Like button can go here
#70 2023-06-10 13:52:25
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
33.7 kilowatt hours for a gallon x 2 equals 67.4 kwh or even more with 1 more gallon at 101.4kwh for a round trip...
gas engine of 1.4 liter size getting roughly 32% efficiency
dumping 45 kw out of the cooling radiator and out the exhaust losing another 120kws.
Does anyone else see a problem with equivalents.
With driving in both direction giving nearly double that to store into sand and then attach to a power creation system at home means I have excess power being saved from just driving to and from work.
Could we even pipe the exhaust heat into this before exiting to save the trips energy that is really hot once coming out of the engine.
How to Calculate Heat Absorption
Calculate heat absorption using the formula:
Q = mc∆T
Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and ∆T is the change in temperature
Offline
Like button can go here
#71 2023-06-11 14:32:15
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Offline
Like button can go here
#72 2023-06-13 21:08:39
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
33.7 kilowatt hours for a gallon x 2 equals 67.4 kwh or even more with 1 more gallon at 101.4kwh for a round trip...
gas engine of 1.4 liter size getting roughly 32% efficiency
This would be for a 60 mph travel speed and of course the bounds were for 30 mph for the hour so we can go with 33.7kwhr or 1 gallon of fuel,
here is a better source
Thermal balance in internal combustion engine with gasoline 2021-36-0025
The results showed that, concerning the energy rate released in the gasoline combustion process, approximately 27% was converted to thermal efficiency (shaft power), 16% was dissipated to the cooling system, 29% was rejected to the exhaust system and approximately 32% was energy lost to the environment, incomplete combustion and lubrication system.
this changes the values of equivalent and seem to fix the conversion errors of the other source.
27% or 9.1 kwhr goes to the wheels
16% or 5.4 kwhr goes into radiator
29% or 9.8 kwhr goes out the exhaust
32% or 10.7 kwhr goes into wasted fuel as its unburned and engine radiated heat
round up error gets to 35 kwhr but close enough.
the desire to use a 20kw motor is just right as that reduces motor losses due to cooling requirements even thou most are liquid cooled at that value.
Mass of radiator can be smaller to reduce mass of the vehicle and coolant amounts if desired.
no engine so no loss of that value just the loss of the container that holds the limestone which is stated by Caliban as being mentioned in post 68 above in quotes.
Engine fuel loss will be zero so we just gained in efficiency and end goal mileage for a heat charged value.
Looks like the desired energy of the loop changes now with heat and cooling response.
So, we need the length of the loop for when the car is off so as to know the number of moles and pressure that we have in each leg of the loop.
post 54 image
typical car length is less than 16 ft from bumper to bumper.
if its front wheel drive that means the generator needs to be in the engine compartment as well as the pump. So, the loop is less than 40 ft even with bends and placement.
We do desire some battery storage, but it does not need to be at motor voltages but at circuit control values for the key ignition and speed monitoring systems.
Any for the motor circuit is to allow for peak speed changes and not for steady state so its a buffer for ripple current changes.
Offline
Like button can go here
#73 2023-06-14 10:19:49
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 30,284
Re: Limestone-based Thermal Battery
After building the power generation thermal limestone battery and testing one will need a donor vehicle to transplant it into to make use of.
Whether this is a cheap junk of old age or a much newer crash repairable is just the means to an end.
Such a vehicle will be a hybrid such that we have all of the existing support after removing the engine and gas tanks to convert into the new transport for human use. This would allow for minimal alterations to prove out the total concept.
This makes for a road worthy means to travel but to make use of it on a highway means that we need a minimum speed of 45mph to which by using an oversized electric motor of 20kw makes this possible with software changes in the computer than normally makes the hybrid change from electric mode to gas powered as we are removing the engine its a needed modification.
Offline
Like button can go here
#74 2023-06-15 04:55:12
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
An alpha stirling engine may be our easiest option.
https://en.wikipedia.org/wiki/Stirling_engine
For small engines, thermal efficiency is between 15-30%. This beats the efficiency we could expect from a small condensing steam piston engine and the stirling will not suffer the same corrosion problems. To achieve good efficiency from a steam cycle, we would need to be able to build steam turbines from high alloy steels. I think this is beyond the capabilities of a home workshop.
Stirling efficiency is still low compared to batteries. But if we can capture wasted heat in stored hot water for later domestic use, the overall exergy efficiency will be competitive. If we assume a heat to power efficiency of 20%, and a COP of 3 for converting electricity into domestic hot water, the effective exergy efficiency of a stirling powered car would be 47%. If on the other hand, the hot water provided by a vehicle displaces a resistance heater, then very little exergy is wasted.
My original estimate was that a 1m sphere of limestone would weigh 1.4 tonnes. This will be towed on a trailer behind the vehicle. Heated to 520°C, it would store some 194kWh of heat. If heat is converted to mechanical power at 20% efficiency, then total work energy available would be 38.8kWh. How much range can we expect? I am going to assume that our vehicle is an SUV like the Ford Explorer.
https://en.wikipedia.org/wiki/Ford_Explorer
This has a curb weight of about 2000kg. Adding the weight of the thermal store, trailer and engine, would increase this to ~3500kg. I am going to ignore air resistance for low speed driving and assume a rolling resistance of 0.01 for rubber wheels on asphalt. The work energy required to drive 1km would be:
Q = 3500kg x 9.81 x 0.01 x 1000 = 343.35KJ (~0.1kWh/km).
Some 38.8kWh of available work energy, would give the vehicle a maximum range of 388km.
Assuming an electricity cost of $0.2/kWh, the energy cost of driving would be $0.1/km or $0.16/mile. However, each kWh used for charging, would provide some 0.8kWh of hot water to the family home. Assuming this would otherwise be provided by heat pump with a COP of 3, each km driven would displace some 0.13kWh of electricity consumption from home hot water heating. So adjusted energy consumption of the vehicle would be 0.37kWh/km, which equates to an energy cost of $0.073/km or $0.118/mile.
These range and energy consumption figures are based upon a vehicle that drives continuously, at low speed over flat ground. The reality is that vehicles must brake for traffic and will need a lot more engine power to drive up hills. Driving on flat ground at 50mph (22m/s), the required engine power would be:
W = m x g x Crr x V = 3500 x 9.81 x 0.01 x 22 = 7,554 watts (10HP).
If we assume that the vehicle needs enough engine power to ascend a 30 degree slope at 30mp (13.4m/s), then minimum engine power would be:
W = m x g x V x sin30 = 3500 x 9.81 x 13.4 x 0.5 = 230.2kW (313HP).
To achieve a range that is close to 388km under real driving conditions, the vehicle must have efficient braking energy recovery. For this reason, my assumption is that the trailer carrying the thermal store and engine, will transfer power to the vehicle wheel via hydraulic transmission. Braking energy will be captured within a hydraulic accumulator and used for launch assist when the vehicle accelerates. This mode of energy recovery is 80% efficient, vs 40% efficient for battery based regenerative braking.
How do prices compare to diesel? In the UK, 1 litre of diesel, containing 10kWh of stored energy costs £2, or $2.4/litre. Each litre of diesel will deliver 3kWh of work energy if burned in a 30% efficient diesel engine. Using the same energy use data as we calculated above, our SUV would have a fuel cost of $0.08/km or $0.13/mile under perfect driving conditions. That works out at 70mpg. So our electric powered SUV would cost about the same to operate as diesel powered SUV.
Last edited by Calliban (2023-06-15 05:50:21)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here
#75 2023-06-15 06:00:22
- Calliban
- Member
- From: Northern England, UK
- Registered: 2019-08-18
- Posts: 4,291
Re: Limestone-based Thermal Battery
Oops! I forgot to include the mass of water in the mass calculations for the car. Assuming that we start with water at 20C and heat it to 100C, then we would need some 1,663kg of water. That would increase the mass of the car from 3.5te to 5.1te. A thermal store with mass 0.7te, water tank with mass 0.8te and trailer and engine with mass 0.2te, would add 1.7te to the mass of the vehicle. That is an increase from 2te to 3.5te, consistent with the estimates above. But maximum range for this vehicle would be 194km, not 388km. Realistic range would be somewhat less. If we assume that:
1. Air resistance is about 33% of rolling resistance;
2. The vehicle loses half of total work energy into braking energy, but recovers 80% of that;
…then actual range on flat ground would be 131km (80 miles).
OK if you want a vehicle that will take you to destinations 30 miles from you front door. For longer trips, not so good.
Larger vehicles, like buses, coaches, ships and trains, can do better. Larger engines are more efficient, larger vehicles have lower drag resistance per unit enclosed volume and buses and coaches have less weight weight per passenger carried. So a stored heat powered bus could realistically transport passengers over distances of hundreds of km. The Alexander Dennis Enviro 500, has a gross weight of 26te and will seat 77 people.
https://www.alexander-dennis.com/media/ … europe.pdf
That equates to a mass of 337kg per passenger. So realistically, a coach powered by a stored heat Stirling engine could get 3x the range that I calculated for the SUV, approximately 400km or 250 miles. This would work for long distance travel.
Last edited by Calliban (2023-06-15 06:25:02)
"Plan and prepare for every possibility, and you will never act. It is nobler to have courage as we stumble into half the things we fear than to analyse every possible obstacle and begin nothing. Great things are achieved by embracing great dangers."
Offline
Like button can go here