New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#76 2022-11-29 08:34:45
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
Design of a balloon borne instrument package for the surface of Venus will necessarily include design of the space descent subsystem. That subsystem might include a surface contact deployment subsystem.
What came to mind, following presentation of the non-flexible nature of ALON(r) as we presently understand it, is that the walls of a buoyancy chamber can be flat, since there is anticipated to be no (or very little) difference in pressure between the interior and the exterior of the buoyancy chamber.
If the walls of the buoyancy chamber are flat, then they can be shipped form Earth flat. At the surface of Venus, while the deployment package endures the temperatures of the surface that will soon cause it to cease to operate, it can lift the walls of the buoyancy chamber into place and fill the chamber with whatever gas was shipped from Earth to start the on site phase of the deployment.
Once released, the instrument package needs to replenish any gas that escapes from the buoyancy chamber with fresh Oxygen extracted from the local atmosphere.
(th)
Offline
Like button can go here
#77 2022-11-29 11:11:34
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,351
Re: Balloon to survive at ground level in Atmosphere of Venus
tahanson43206,
Even for your Earth-bound hot air balloon example, we have things going on inside the balloon that adjust temperature and therefore gas pressure, which is why a hot air balloon is buoyant within Earth's atmosphere. 30km above the surface of Venus, we're going to thermal soak to 220C (conventional oven baking temperatures). A BNNT fiber reinforced thin PTFE tape can survive the heat and acid, but not the temperatures much below that altitude, so 30km is about as close to the surface as we can get without utilizing much more complex to make and therefore expensive materials. At 30km, we also have 35km/s wind speeds and 9.85X greater atmospheric density than we do at Earth sea level.
Offline
Like button can go here
#78 2022-11-29 11:22:38
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
This topic is about design, manufacture, shipping and deployment of a balloon supported instrument package for exploration of the surface of Venus.
The conditions present in that environment are so extreme that innovation is required to achieve the objective. Fortunately, NewMars forum of the Mars Society is a place where innovative thinking and practical knowledge jostle each other in a process that often leads to understanding of new possibilities.
(th)
Offline
Like button can go here
#79 2022-11-29 20:54:27
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,933
Re: Balloon to survive at ground level in Atmosphere of Venus
Here is the lift equation of air being warmed for a given lift
https://www.brisbanehotairballooning.co … loons-fly/
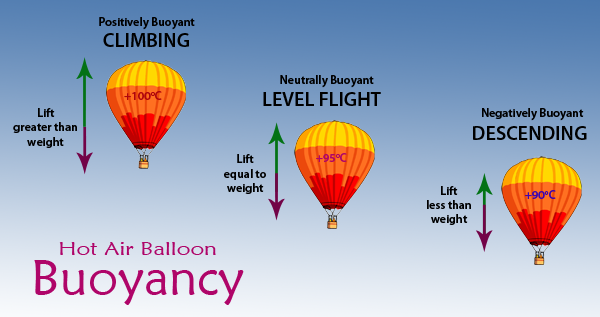
https://www.engineeringtoolbox.com/hot- … d_562.html
Hot Air Lifting Force
The lifting force from a hot air balloon depends on the density difference between balloon air and surrounding air, and the balloon volume. The lifting force can be calculated asFl = V (ρc - ρh) ag (1)
where
Fl = lifting force (N, lbf)
V = balloon volume (m3, ft3)
ρc = density cold surrounding air (kg/m3, slugs/ft3)
ρh = density hot balloon air (kg/m3, slugs/ft3)
ag = acceleration of gravity (9.81 m/s2, 32.174 ft/s2)
Offline
Like button can go here
#80 2022-11-30 08:59:44
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re Post #79
It may be helpful to a mission planner, to have available the information you have posted here.
In the case of the Venus surface exploration balloon supported instrument package, the lift (as currently envisioned) is provided by the difference of the mass totals for a volume of Oxygen inside an envelope surrounded by an atmosphere of CO2.
GW Johnson has introduced to this topic the possibility of having an open vent at the bottom of the lift envelope, as you have shown in your post.
The gas inside the envelope is Oxygen (or another lighter mass gas).
If the gas is allowed free exchange with the CO2 atmosphere, it seems to me reasonable to suppose that the two gases would intermingle, and the lifting properties of the Oxygen would be lost.
For this reason, I am skeptical that the open vent idea is appropriate for the conditions at the surface of venus.
As a reminder.... The temperature at the surface of Venus is in the range of:
It appears that the surface temperature ranges from about 820 degrees to nearly 900 degrees F. The average surface temperature is 847 degrees F., hot enough to melt lead.
The Planet Venus - National Weather Service
www.weather.gov › fsd › venus
A hot air balloon might work on Venus, provided that the working fluid is CO2, and temperatures inside the envelope are greater than temperatures outside.
I am wondering if you have time to explore the potential, based upon the information you have provided.
You would (appear to) have the ingredients you'd need to perform the calculation.
You'd need to match the lift of Oxygen, to make the exercise worth while... 17 kilograms per cubic meter is the lift of Oxygen at the surface of Venus.
The calculation should be (hopefully) straight forward... You have a volume to work with (one cubic meter)
You have the outside temperature (you can chose from 820 through 900 degrees Fahrenheit)
You can use Earth's gravity (because it's close to that of Venus) or if you are interested in accuracy you can go with Venus gravity:
Per Google:
Venus / Gravity
8.87 m/s²Venus
Planet
You'll need to determine the density of CO2 at 90 bar and the temperature you have chosen.
With that information, you'll (hopefully) be able to determine the density needed inside the envelope to achieve 17 Kg of lift.
Finally, with ** that ** density available, you should be able to discover the temperature needed inside the envelope to achieve it.
Whatever temperature you find, that temperature must be produced by a heat source under the envelope, such as a small nuclear reactor, perhaps.
The method seems possible, but definitely a stretch for engineers asked to make equipment to operate in this mode at the surface of Venus.
(th)
Offline
Like button can go here
#81 2022-11-30 21:12:12
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,933
Re: Balloon to survive at ground level in Atmosphere of Venus
This article shows how to power a camera from sound waves.
This sound-powered underwater camera could dramatically boost climate monitoring
/2022/11/29/image/png/ByDenrTMsZF2bUvrbyR0Vg0wRy4mneek8ZUw6Qyj.png)

Offline
Like button can go here
#82 2022-12-01 08:04:23
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re #81
Thank you for the post about possible energy production by harnessing sound wave action. Sound waves (as I understand them) are experienced as compression and decompression of molecules as waves proceed through a gas, as compared to the kind of waves harnessed in an ocean surface situation.
For clarification of this point, if a member of the forum has time, it would be interesting (to me for sure) to see how sound waves manifest in a gas mixture.
Microphones depend upon sound waves, and movement of a metal diaphragm with respect to a permanent magnet is one of several ways to "measure" sound waves in a gas mixture. In fact (come to think of it) a microphone ** is ** a means of capturing energy from sound waves.
Likewise, a loud speaker cone advances and withdraws as it translates electrical input to a coil of wire that is itself in a permanent magnet field, into mechanical motion of gas molecules adjacent to the cone.
(th)
Offline
Like button can go here
#83 2022-12-01 08:09:43
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
In a related topic, kbd512 reminded forum readers that gliders may prove to be a useful tool for exploration of Venus, which has robust winds compared to Mars.
In an article about a proposed glider design for Venus, the author included an image (artwork) of an artist's imagining of what the surface of Venus might look like.
While the artwork came across to me a fanciful, I ** did ** pick up on the hint there may be lightning in the environment below and perhaps inside clouds on Venus.
If that ** is ** case, then lightning would be a hazard for flying vehicles in the atmosphere of Venus, and potentially for ground vehicles.
On the other hand, a difference of electrical potential ** might ** represent a power generating opportunity for equipment deployed to Venus.
The risks may far outweigh the potential benefits, but this ** is ** the NewMars forum, where extremes are routinely considered.
(th)
Offline
Like button can go here
#84 2022-12-01 16:19:43
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
The plot thickens.... Sulfur plays more of a role in the lower atmosphere than I had realized ...
https://space.stackexchange.com/questio … h%20higher.
The atmosphere of Venus up to 48 km comprises high velocity jetting of sulfur (SN) gas through over 1,000,000 ‘small domes’ imaged by Soviet and NASA Magellan radar. SN gas, the normal form of sulfur at the temperature in the lower atmosphere, jets at high speed until it reaches the altitude (temperature) at which it crystallizes to form a monoclinic crystal and due to the heat released rises a few km to the temperature level where it changes to a rhombic crystal. The denser rhombic crystals fall downward and revert to the monoclinic form which, the change of which is completely reversible. This produces the the thin roiling lower cloud layer, the mass of which, along with the jetting SN and CS produce the high pressure at the surface.
The Lower Cloud Layer (LCL)forms the boundary between the two modes of Venus’ bi-modal atmosphere, where different molecular species and pressure altitude relationships attain. Changes in molecular species with elevation can only occur in such a ‘mass flow’ environment. The original Pioneer Venus figure showed the physical layers and temperatures of each as a function of altitude, but the chemical composition above and below the LCL were not be determined.
A three order-of-magnitude drop in the Pioneer Venus CO2 mass spec channel at the LCL altitude was interpreted as a clogging of the inlet leaks (there were two at the time). The mass 44 counts remained low until PV descended to 31 km, the bottom of the red-haze layer, where the channel 44 counts recovered. This was attributed to the evaporation of the occluding droplets and the counts below 31-km were assumed to be CO2. The crystallization temperatures of monoclinic and orthorhombic S8 correspond exactly with the temperature at the LCL. The mass spectrometer not detect the S8 because the mass of the S8 molecule, 256.47 amu is beyond the range of the mass spectrometer instrument, 208 amu.
Carbon Sulfide, CS, occupies the entire lower atmosphere of Venus along with S8. What scientists expected to be CO2 below 31 km is actually CS (carbon sulfide), ‘masquerading’ as CO2. CS and CO2 have very similar masses – 44.0686 and 44.0096 amu, respectively. The evidence indicating CS in the lower atmosphere is present in the form of the red haze observed from 31 km up to the LCL. Again, a simple look-up of the temperature at which rising hot CS gas would form small red crystals (200 C) coincides exactly with the lower level of the red haze.
ref: http://firmament-chaos.com/papers/Venus-Paper-2018.pdf
edited Oct 21, 2019 at 21:32
Brian Tompsett - 汤莱恩's user avatar
6,13333 gold badges2727 silver badges4747 bronze badges
answered Oct 20, 2019 at 2:01
John Ackerman's user avatar
Following up later .... the compound SN is identified in the quote above:
Sulfur mononitride - Wikipedia
https://en.wikipedia.org › wiki › Sulfur_mononitride
Sulfur mononitride is an inorganic compound with the formula SN. It is the sulfur analogue of the radical nitric oxide, NO. It can be produced through ...
What I'm trying to discover is what effect (if any) the compounds identified in the quote above might have on ALON(r).
Following up further:
https://www.washington.edu/news/2021/01 … venus-so2/
The article at the link above covers research and analysis that supports the view that Sulfur dioxide may be responsible for radio signals that were thought (for a time) to be phosphine.
“Instead of phosphine in the clouds of Venus, the data are consistent with an alternative hypothesis: They were detecting sulfur dioxide,” said co-author Victoria Meadows, a UW professor of astronomy. “Sulfur dioxide is the third-most-common chemical compound in Venus’ atmosphere, and it is not considered a sign of life.”
The team behind the new study also includes scientists at NASA’s Caltech-based Jet Propulsion Laboratory, the NASA Goddard Space Flight Center, the Georgia Institute of Technology, the NASA Ames Research Center and the University of California, Riverside.
The UW-led team shows that sulfur dioxide, at levels plausible for Venus, can not only explain the observations but is also more consistent with what astronomers know of the planet’s atmosphere and its punishing chemical environment, which includes clouds of sulfuric acid. In addition, the researchers show that the initial signal originated not in the planet’s cloud layer, but far above it, in an upper layer of Venus’ atmosphere where phosphine molecules would be destroyed within seconds. This lends more support to the hypothesis that sulfur dioxide produced the signal.
It seems likely that Sulfur Dioxide and Carbon Dioxide are present in the atmosphere of of Venus, near the surface, where the proposed balloon instrument package would attempt to navigate.
(th)
Offline
Like button can go here
#85 2022-12-01 19:41:15
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,351
Re: Balloon to survive at ground level in Atmosphere of Venus
AZO Materials - Transparent Aluminum (Aluminum Oxynitride) – Properties, Production and Applications
Innovacera Technical Ceramic Solutions - Aluminum Nitride Properties And Applications
Edit (tahanson43206, I beliee this link has a compatibility chart containing the specific chemical compounds you're interested in):
Compatibility Manual - Liquids–Metals–Elastomers–Plastics
Last edited by kbd512 (2022-12-01 20:32:14)
Offline
Like button can go here
#86 2022-12-02 14:52:29
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,518
Re: Balloon to survive at ground level in Atmosphere of Venus
For kbd512 re #85
Thank you for another substantial contribution to this topic! Once again, I'll have to schedule time to follow the links this weekend. They definitely look promising!
I've stopped off in this topic to bring a recent post by Calliban into focus:
http://newmars.com/forums/viewtopic.php … 53#p203853
While I have not yet followed the link provided by Calliban, I am trusting his announcement of the work on an improved catalyst to make Carbon Monoxide.
The production of Carbon Monoxide is of interest for manufacture of synthetic carbon fuels on Earth, and it very well may be of interest on Mars and on Venus for that purpose.
However, I am more interested in the production of Oxygen from the vast quantities of CO2 available in the atmosphere of Venus. An improved catalyst implies (to me at least) that less energy is needed to liberate Oxygen to replace any that is lost in the course of operations on Venus.
(th)
Offline
Like button can go here
#87 2022-12-02 20:39:51
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,933
Re: Balloon to survive at ground level in Atmosphere of Venus
The breakdown of co2 into o2 and co is an ongoing experiment in that it would proving the much-needed oxygen for man to survive on mars in the test article called Moxie.
I do believe that this is a product for a Venus near surface probe to be able to get high quality images back to earth for lots of science to be had from them.
Offline
Like button can go here
#88 2023-03-19 06:13:11
- Mars_B4_Moon
- Member
- Registered: 2006-03-23
- Posts: 9,776
Re: Balloon to survive at ground level in Atmosphere of Venus
Potentially Active Volcanoes Have Been Found on Venus
Offline
Like button can go here