New Mars Forums
You are not logged in.
- Topics: Active | Unanswered
Announcement
#26 2022-11-20 21:41:39
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re #24
The focus of this topic has been the surface.
The idea of floating a "normal" balloon in the upper atmosphere has been explored by NASA and others.
The current topic is intended to follow the idea of Void, to operate at the surface.
It appears that the idea has merit. One detail that seems squishy is the temperature.
you used 700 Centigrade and I've been using Fahrenheit ....
Here is a quote that may help to clarify the temperature ...
It appears that the surface temperature ranges from about 820 degrees to nearly 900 degrees F. The average surface temperature is 847 degrees F., hot enough to melt lead.
Sidereal Rotation: 243 Earth days
Atmosphere: Carbon Dioxide 96.5%, Nitrogen ...
Length of Day: 116.75 Earth daysThe Planet Venus - National Weather Service
https://www.weather.gov › fsd › venus
So by this report my estimate of 700 Fahrenheit has been low ...
Never-the-less, the suggestion of kbd512 to use ALON seems like a definite winner, because electronics inside the balloon could take pictures.
You had expressed concern about stability of the balloon, but I think that simply mounting the electronics and battery in the inside wall would stabilize the vehicle.
Delivery of a good sized experiment package should be possible from orbit, by using a parachute after heat shield discard.
On Venus there is plenty of atmosphere to slow descent.
(th)
Offline
Like button can go here
#27 2022-11-20 21:51:24
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
Mean surface temperatures on Venus are near 460C, not 700C.
GaN-on-diamond semiconductor material that is stable to 1,000C
If we have microchips that can operate at up to 1,000C, that means simple purpose-built electronics operating at half that temperature are doable, which means lasers, cameras, radios, memory cards, etc, are also operable. Silver melts at 962C. Copper melts at 1,085C. ALON melts at 2,150C. BNNT fabric is also good to about 1,000C in an oxidizing atmosphere, even if its melting point is technically 2,970C.
This is a very challenging technical proposition, but still doable.
Offline
Like button can go here
#28 2022-11-20 22:11:13
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
The atmosphere does not completely slow anything heading to the ground and that is why we use parachutes and heat shield along with retro rockets to perform the final breaking to the ground since there is no ocean to splash down into. The glass will not float fast enough to slow its eventual impact. Releasing the floating ball on orbit means that it will not fall to the ground at all but will escape the planets gravity due to orbiting velocity of entry to Venus's orbit. This means we must bring it downward towards the planet before releasing it and it is going all that speed of momentum to have the buoyancy to resist that with.
On the surface there is no buoyancy it's a rock...
https://www.omnicalculator.com/physics/buoyancy
Offline
Like button can go here
#29 2022-11-20 22:39:15
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re #28
You're close but not yet quite at the point of this topic....
This topic is about a balloon, not a rock.
The buoyancy is due to the difference of molecular weight of oxygen molecules as compared to carbon dioxide.
There are posts in this series that include specific facts about the relative molecular weights of the two materials.
Even at the surface of the Earth, there appears to be 600 grams of lift available from floating oxygen inside a sphere with CO2 outside,
On Venus, while I have not yet seen any computations from any NewMars member, I am guessing the ratio of lift will be similar.
I don't understand why you seem to think the balloon is going to have any velocity toward the surface to speak of. The atmosphere of Venus is thick, and it gets thicker the further down you go.
The balloon package should be able to release from the parachute well above the surface.
If the shell is made of ALON as kbd512 suggested, then it can be both thin and able to vent gas if there is a need to do that, or ballast if there is a need to climb.
It seems to me the idea of Void has a realistic chance of success in the Real Universe, so it is time to nail down the unfinished computations identified in this topic, and then to seek an avenue for publication of the idea outside the small readership of this forum.
(th)
Offline
Like button can go here
#30 2022-11-21 01:52:09
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
CO2 at STP is 1.96g/L.
O2 at STP is 1.43g/L.
He at STP is 0.1786g/L.
1L of pure O2 at STP provides a buoyancy / lifting force of 0.53g in a pure CO2 atmosphere.
1L of pure He at STP provides a buoyancy / lifting force of 1.7814g in a pure CO2 atmosphere.
At 92 Bar and 462C, CO2 is 66.25g/L.
I don't know exactly what the density would be for He at 92 Bar, but somewhere between 1.3g/L to 1.4g/L, so 64.85g of lifting force.
A regulation baseball is 207cm^3, or 0.207L, so only 13.42395g of lifting force.
A miniature laser pointer can weigh as little as 1g, we would factor in another 1g for the electronics, 1g for its nuclear battery, and 1g for a BNNT streamer loop.
That only leaves 9g for the ALON shell.
I don't see how this is going to work at all if the goal is to get the camera / laser mapper near to the surface.
You're much better off creating a solid sphere of ALON, roughly the size of a "big masher" marble, putting the camera or laser inside that, attaching it to a sufficiently long BNNT streamer loop for the wind currents to act on, and then those convective currents will cause it to drift for many many miles before it finally impacts the surface.
A 25mm ALON sphere with laser / electronics / nuclear battery / BNNT streamer should weigh about 35g. It's not going to "float", but it will take quite some time to finally drift downwards and impact the surface.
Offline
Like button can go here
#31 2022-11-21 08:35:22
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For kbd512 re #30
Thanks for showing the numbers for 1 liter volumes, and for a baseball sized probe.
Where did that size come from?
There is no reason (that I can think of (but there may be one)) why a probe needs to be that small.
I've been thinking along the lines of one cubic meter for calculations, which would be a sphere .621 meters in radius (per gigacalculator.com)
If you increase the size of your probe from baseball to one cubic meter, everything should improve markedly.
You'll have more lift for the ALON shell, and since there is no pressure difference between inside and outside, it is not doing any work other than keeping molecules apart.
At that size, there may (hopefully) be some mass to play with to achieve useful objectives, such as navigating vertically to take advantage of wind currents, as free flying balloons do on Earth.
Thanks again for the array of precise numbers to work with.
(th)
Offline
Like button can go here
#32 2022-11-21 09:24:47
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
With the small size under even a meter we can pack a payload shroud or fairing with many of these to be released once well into the atmosphere to where they can spread out once released to be carried apart by the winds of that region of the planet. The natural effect of a parachute of BNNT would slow the ball to that buoyancy point. Is it intended to keep that parachute as it becomes a sail to move and give uplift with wind most of the time as it could lose the up draft and cover the ball.
What are the highest mountains to stay above so as to get most out of the optics as it moves around the planet?
Offline
Like button can go here
#33 2022-11-21 12:25:36
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
tahanson43206,
Small probes can be carried by the hundreds. The smaller / ligher a probe is, the less it costs. We can afford to have more of them, for more mapping missions to more planets. There's also a much better chance of obtaining some usable data from hundreds of smaller probes. Single points of failure are generally bad. Recall that this is ultimately a high-precision mapping mission, similar to the one performed by the Mars Reconnaissance Orbiter. We have relatively low precision maps of the surface of Venus. If we did send a rover down to the surface, then landing it somewhere other than a lava puddle would be a good idea.
Increasing the size of the probe won't help. That is true 100% of the time as it relates to material strength. A 1 pound bar of steel 12 inches long is [Edit: "stronger", NOT "weaker"] than a 1 pound bar of steel 24 inches long. Tensile strength is a fixed value for any given material, at a specified temperature. The tensile strength of quartz or ALON is therefore, a fixed value. As the bubble gets bigger, it also gets weaker for equivalent weight.
This is also why the solar powered airplane doesn't work. The tensile strength of CFRP doesn't improve by building a longer / bigger wing- it only gets weaker for a given scaled-up size. You need an Airbus A380 sized wing to carry 1 person aloft using photovoltaics and batteries, and then the wing was / is / ever shall be so close to its structural limit, aka "rapid unplanned deconstruction", that it had to be put in a museum after its one and only flight around the world, which lasted for over a month versus less than a week in the case of Burt Rutan's Voyager. Improve the tensile strength by 4X, and congrats, you now have a solar powered airplane that does what a Cessna 182 does, apart from only flying well below the Cessna 182's stall speed, which costs as much as a jet airliner like the Boeing 737, but only lasts for 1 lousy flight.
So, while I can appreciate the belief that "building it bigger" provides more lifting force, the fact of the matter is that quartz and ALON are both too heavy to use as a protective shell, assuming the goal is to get this thing to "float" near to the surface. Higher up in the atmosphere is better on account of temperature and pressure reduction, but then you also have less atmospheric density to work with, so that still doesn't help you very much.
Make the probe the size of a large marble, put a sufficiently long / light / high temperature resistant ribbon streamer on it to catch the thermals, and call it a day. This doesn't work well enough any other way. It's a fun idea to think about, though.
Last edited by kbd512 (2022-11-22 16:13:04)
Offline
Like button can go here
#34 2022-11-21 19:32:54
- Void
- Member
- Registered: 2011-12-29
- Posts: 9,001
Re: Balloon to survive at ground level in Atmosphere of Venus
(th) and others, please review this post, "Index» Terraformation» Worlds, and World Engine type terraform stuff."Post #627. Thanks.
Is it possible that the root of political science claims is to produce white collar jobs for people who paid for an education and do not want a real job?
Online
Like button can go here
#35 2022-11-21 21:49:30
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
This appears to be a good time to call this topic finished.
I am satisfied that the concept is valid, and appreciate all the valuable input contributed by NewMars members.
(th)
Offline
Like button can go here
#36 2022-11-22 15:14:31
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
This topic reminds me of the classic egg drop in that we want it on the ground before we can truly establish floating since we need to get rid of the parachute that could cover it or not all enough aero speed breaking so as to keep it from going smack into the ground. Normally it is cradled in a can that has foam to act as a cushion to absorb the impact of the sudden stop.
We do have a very tough sphere but the contents inside are less so and are considered fragile under such shock waves. Sure, we do know of military grade products that could make this less fragile but getting that will be hard without a good thought-out plan to provide why it's needed.
We know that past design would survive long enough to land and then release the sphere to allow for what we would want to do.
Offline
Like button can go here
#37 2022-11-22 19:42:33
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
This is a follow up question for kbd512 if you happen to catch it ...
In Post #30, you gave two values for CO2
CO2 at STP is 1.96g/L.
At 92 Bar and 462C, CO2 is 66.25g/L.
I am unsure how you got to the 92 Bar/462C figure, and was hoping you might show a hint about the process.
Thanks again for the numbers in Post #30.
Update a bit later .... after fooling around with the online calculators for a while, I finally deduced that it is possible to compute the lift on Venus.
Given molecular weight of O2 molecules as 32 g/mol and
Given molecular weight of CO2 molecule as 44 g/mol
It is possible to confirm that the lift per mol is 12 grams.
This lift is independent of temperature and pressure, as long as the two gases are at the same values.
I then asked (in this case: gigacalculator.com) to show me how many mols of gas would be contained in a volume of 1 cubic meter, at 90 bar and 462 C.
The result turned out to be 1472.4.
This means that the lift available for a 1 cubic meter volume of O2, in an atmosphere of CO2, would be 1472.4 * 12, or 17,668.8 grams, or 17.67 Kg.
Now the thickness of the ALON shell needs to be no greater than the thickness to prevent O2 molcules from drifting through the wall to mix with the CO2.
In my scenario, the temperature inside the vessel is the same as the temperature outside, and the pressures are identical.
In other words, the wall is NOT containing anything except brownian movement of O2 molecules.
Since we have 17+ Kg to work with, I am expecting there is plenty of mass available for equipment and (high temperature) battery, after the mass of the (very thin) shell is allocated.
What is more, in the scenario I am offering, there is NO reason the probe has to be limited to a volume of 1 cubic meter.
The volume can be as large as will fit in the upper stage of the launch vehicle, behind the heat shield and parachute package.
I see no need to keep the size of the probe any less than will fit in the launch vehicle.
I'd appreciate kbd512 (or anyone with an interest in this topic) to double-check my computations (or rather, my transcription of the online calculator output).
Touchup a bit later:
Question: How large would be a spherical probe with volume of one cubic meter?
Per online Sphere Calculator:
Sphere Calculator
Choose a Calculationr, C, A | Given V
volume V =
1
Let pi π =
3.1415926535898
Units
m
Significant Figures
9
Answer:
radius r = 0.620350491 m so diameter = 1.24 meters
volume V = 1 m3
surface area A = 4.83597586 m2
circumference C = 3.89777709 m
The volume of the weather balloon shown below is about 4 cubic meters.
Update next day .... the weather balloon shown is 78 inches in diameter (per web site) or nearly 2 meters.

Choose a Calculation
A, V, C | Given r
radius r =
1
Let pi π =
3.1415926535898
Units
m
Significant Figures
9
Answer:
radius r = 1 m
volume V = 4.1887902 m3
surface area A = 12.5663706 m2
circumference C = 6.28318531 m
That would imply that the lift of that sphere, holding Oxygen in an atmosphere of CO2, at 90 bar and 462 degrees Centigrade would be 4 times 17 or more than 68 kilograms.
I'd appreciate someone checking my work. 68 kilograms would support a capable science package.
Reminder: There is NO need to go for exotic atoms for the interior of the probe. Oxygen is readily available, and it appears to work well.
The lift is 12 grams per mol. To find total lift, find the number of mols in your sphere, and multiply by 12 grams.
(th)
Offline
Like button can go here
#38 2022-11-23 08:43:14
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
For CO2 density, I used an online calculator.
A sphere containing 1m^3 of volume has a radius of approximately 620.35mm, so V = 999997626mm^3.
Let's assume the sphere is about 2mm thick, meaning radius is now 622.35mm, so V = 1009700780mm^3.
Outer sphere diameter is therefore 1,244.7mm or about 1.245m.
1009700780mm^3 - 999997626mm^3 = 9,703,154mm^3 = 9,703.154cm^3
ALON is 3.7g/cm^3, so 3.7 * 9,703.154 = 35,901.6698g
Quartz is 2.65g/cm^3, so 2.65 * 9,703.154 = 25,713.3581g
Pyrex is 2.23g/cm^3, so 2.23 * 9,703.154 = 21,638.03342g
1kg = 1,000g, so the ALON sphere weighs 35.9kg, the Quartz sphere weighs 25.7kg, and the Pyrex sphere weighs 21.6kg.
You said you had 17kg of lifting force to play with, using O2 as your lifting gas.
ALON has a tensile strength of 631MPa at 500C, or 91,518.8psi. 92bar is 1,334.35psi. So I guess we're good there, except that
If this object is supposed to be a glass balloon containing a camera (which presumably has some weight to it, can you identify any potential problems with this idea?
Can you appreciate just how thin a 2mm thick 1.245m diameter sphere happens to be?
That's about as thick as 3 sheets of construction paper (~0.6mm per sheet) and the object in question is 49 inches in diameter.
Can you understand why I wanted to use Helium to pressurize the bubble?
I was looking for any little advantage to make the concept viable.
Finally, can you understand why I abandoned this idea and suggested going with a bunch of miniature cameras inside marbles instead?
It's not because I don't like the idea.
Offline
Like button can go here
#39 2022-11-23 09:12:03
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For kbd512 .... thanks for continuing to work this problem!
I will have to print and study your post, and will make time to do that later today.
The wall of the ALON sphere proposed is 2 mm thick, so it should be possible to discover the total volume of ALON required for the shell.
The mass of ALON is provided, so the total amount of weight should be discoverable.
For some reason I'm not getting through ... there is NO pressure difference between the inside and the outside of the sphere.
I have NO idea where that is coming from, except that the topic may have been distracted early on by thoughts of human beings inside.
This is intended to be a scientific information gathering package, able to survive in the conditions that exist just above the surface of Venus.
We seem to be on a solid track to achieving the performance goals, but I'll have to study your post to be sure.
Thanks again for a boost to the topic!
(th)
Offline
Like button can go here
#40 2022-11-23 09:42:27
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
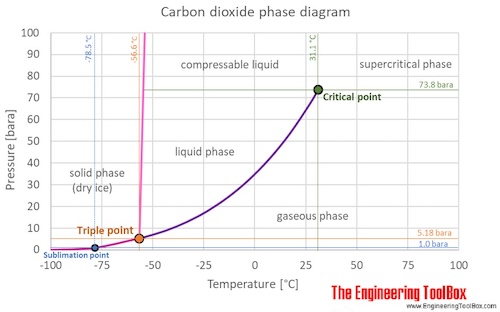
The sphere thickness is not just the location to which it must land at for temperature and pressure but be capable of handling other combinations of them from launch through to the delivery of it to the Venus near zero external atmospheric pressure at entry until it gets to where we want to have it end up at.
Here is the shift for carbon dioxide inside the ball rather than oxygen
https://www.engineeringtoolbox.com/CO2- … _2017.html
Offline
Like button can go here
#41 2022-11-23 11:51:47
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
tahanson43206,
Seriously? I did "discover" both the total volume and weight of the ALON shell.
Volume of a sphere is 4/3 * π * r^3
π = "pi"
pi = 22/7, or 3.14
r = radius
radius = 1/2 of diameter
Volume of ALON or Quartz or Pyrex "glass" container material:
1009700780mm^3 (volume of 622.35mm radius sphere, for a 2mm wall thickness "glass" container, in cubic millimeters)
MINUS
999997626mm^3 (volume of 620.35mm radius sphere capable of holding 1m^3 of gas)
EQUALS
9,703,154mm^3 (the total volume of the "glass" material containing the lifting gas, if the container is 2mm thick and it contains 1 cubic meter of internal volume)
1cm = 10mm
1,000mm^3 (10mm Length x 10mm Width x 10mm Height) = 1cm^3
9,703,154mm^3 / 1,000mm^3 = 9,703.154cm^3
ALON has a bulk density of 3.7g/cm^3.
3.7 (ALON bulk density)
MULTIPLIED BY
9,703.154 (2mm wall thickness spherical glass container that holds 1 cubic meter of internal volume to supply 17kg of lifting force when the gas inside is O2 and the gas outside is CO2, expressed in terms of cubic centimeters)
EQUALS
35,901.6698g
1,000g = 1kg
35,900g = 35.9kg
How else do you think I computed the volume and weight?
Now, as to your assertion that there is no pressure differential between the inside and outside of the sphere...
Do you have a Star Trek Teleporter to teleport said glass sphere to the surface of Venus (and if you do then why bother with this to begin with)?
If you cannot answer that question in the affirmative, then I presume said sphere will arrive from Earth sea level 1 Bar of pressure, go through a vacuum with 0 Bar of pressure after being subjected to a lot of g-loading and vibration, and without teleporting somehow makes its way near to the surface where it will float just above the ground, meaning it will transition through an atmospheric density ranging between 0 Bar (in outer space) to 92 Bar (atmospheric pressure near the surface of Venus).
How is said sphere maintaining equalized pressure with the outside atmosphere during its transition to the surface (0 Bar to 92 Bar)?
Where is my magic internal pressure equalization coming from? How is that being provided?
If my 49 inch / 1.245m diameter sphere is buoyant at 92 Bar of pressure, then it won't be buoyant at all at 1 Bar of pressure, which means it will fall as it transitions from a mildly cryogenic temperature in space or the upper atmosphere of Venus to temperatures hot enough to melt Lead. Maybe it can do that if it has enough time to stabilize its internal temperature. All of this conveniently ignores the fact that this sphere is not buoyant at all at 92 Bar of pressure, and if we halve the ALON thickness, then it's still not buoyant at all. Scaling up the size of the sphere increases its "air resistance" so it falls slower, but it's still going straight to the surface and it's not stopping. If I switch to Pyrex and make the sphere shell half as thick (1mm vs 2mm), then I have some usable mass margin to play with, but Pyrex is nowhere near as tough as ALON (resistant to cracking over rapid temperature changes).
Helium would certainly help, but how are we equalizing internal pressure during our wild temperature and pressure transitions?
I know you're interested in this idea, as I was initially before doing some basic math, but it doesn't work well enough. It's right at the edge of feasibility, but on the wrong side of it.
Offline
Like button can go here
#42 2022-11-23 12:20:48
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
As you indicate a 1 bar change in surface pressure inside the sphere will allow for it to float after temperature equalization so as you indicated its going to smack into the surface first and hard.
https://science.nasa.gov/science-news/s … st14mar_1/
A typical window for a house on Earth has 2 panes of glass, each about 1/16 inch thick. In contrast, the ISS windows each have 4 panes of glass ranging from 1/2 to 1-1/4 inches thick. An exterior aluminum shutter provides extra protection when the windows are not in use.
http://www.surmet.com/docs/Product_sheet_ALON.pdf
https://www.engineeringtoolbox.com/oxygen-d_1422.html
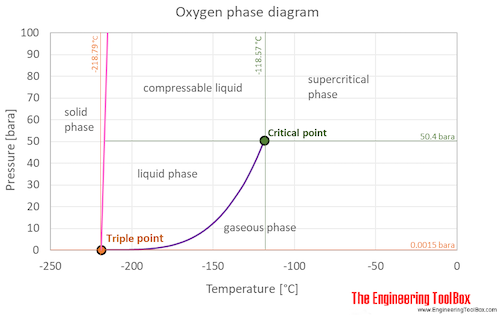
Liquid oxygen has an expansion ratio of 860:1. This means that as it boils off from -297°F to ambient, it expands 860 times its volume
https://ehs.mit.edu/wp-content/uploads/ … OXYGEN.pdf
formula is PV = nRT (n is the amount of gas and R is a constant)
Offline
Like button can go here
#43 2022-11-23 12:22:22
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For kbd512 .... thanks for your continuing interest in and support of this topic ...
I'm looking forward to finding the time to print and study your (now two) posts about computing the mass of the shell using ALON.
There is no point in being impatient, because expressing impatience won't accelerate the process << grin >>
However, ahead of finding that time (which is likely this weekend) I ** can ** help your understanding of what I have in mind, by explaining (patiently) that there is absolutely NO reason WHATSOEVER why the sphere has to be sealed.
Again, I have NO idea where that idea came from, but now that it's on the board, let's remove it.
The envelope of the (proposed) instrument package does NOT have be sealed at any point in the shipment process, except at the last moment, when oxygen is injected into the volume as the delivery package descends and pressure increases.
At the surface, the oxygen contents of the envelope will match the pressure (and temperature) of the outside CO2, and the natural buoyancy of Oxygen in CO2 will provide the multiple kilograms of lift that are needed.
Thanks (again) for your interest in and support of this topic!
It is difficult to collaborate with just text, but (as this forum has shown multiple times) it ** can ** be done!
(th)
Offline
Like button can go here
#44 2022-11-23 12:29:02
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
Not sealing the container means you now have allowed moisture and dust plus to enter where you now need lots of equipment to make it sealed to the level required and that also means you introduced a fracturing point when the internal spheres pressure raises due to the temperature differential that is caused by the atmosphere and landing of Venus.
Offline
Like button can go here
#45 2022-11-23 12:47:08
- kbd512
- Administrator
- Registered: 2015-01-02
- Posts: 8,331
Re: Balloon to survive at ground level in Atmosphere of Venus
tahanson43206,
If the sphere is not sealed, then the lighter gas will escape as it's heated up inside the sphere.
That means you no longer have any buoyancy.
So, what is the point of this?
Offline
Like button can go here
#46 2022-11-23 12:53:00
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re #44
Thanks for pointing out the need to plan for (and to regulate) the gas mixture inside the envelope of the instrument package during preparation for flight, during flight, during descent and upon deployment.
I seems possible (perhaps even likely) that there are ways to insure only wanted atoms enter the space.
On Earth, the contents of the envelope would be ordinary air, such as would be found in a clean room.
As the package ascends to orbit, the air inside the enclosure can be allowed to exhaust. During flight, there is no reason for anything to be present in the chamber, unless the instruments require it for some reason. Hopefully instruments and electronics could be packaged to endure vacuum during flight.
As the package descends, pure Oxygen would be admitted to the envelope as pressure increases. The pressure would be kept just slightly ahead of whatever the external pressure might be.
Thanks again for thinking of and writing about management of gas pressure in this system throughout the phases of preparation, flight, landing and deployment.
(th)
Offline
Like button can go here
#47 2022-11-24 07:14:56
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
In posts recently in this topic kbd512 has proposed a thickness for an ALON shell for the proposed balloon probe for the surface of Venus, of 2 mm.
I am still planning to study the two posts kbd512 has provided, on how to compute the mass of the shell this weekend, in hopes of gaining an understanding of the calculations performed.
However, this post is about the thickness proposed, of 2 millimeters. I think that is much thicker than is needed for this application.
This structure is NOT intended to perform any function OTHER than:
1) Keep Oxygen molecules inside the envelope
2) Keep CO2 molecules outside the envelope
3) Carry the load of the package, which is just under 17 kg for a 1 cubic meter sphere of 1.24 meters diameter.
It appears to me that ordinary household aluminum foil might be a good model for the shell of this device:
How Thick Is Aluminum Foil? Regular aluminum foil, also called standard household or standard duty foil, will typically be around 0.63 mils thick. Some thicker aluminum foils or extra heavy-duty household foils can be up to 0.94 mils thick.
Nov 18, 2021
Thickness of Aluminum Foil: 9 Ways to Use Aluminum Foil - 2022
www.masterclass.com › articles › thickness-of-aluminum-foil
About Featured Snippets
We have a lift of 17+ kg for a cubic meter of Oxygen in an atmosphere of CO2 at 90 bar and 462 degrees Centigtrade.
I am hoping the shell around that volume will mass under a kilogram, and would ask what a thickness of 1 mm would look like?
Can the tensile strength of 1 mm thickness of ALON carry the proposed load of 17 kg?
(th)
Offline
Like button can go here
#48 2022-11-24 09:06:37
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
https://en.wikipedia.org/wiki/Superpressure_balloon
The issue for shell thickness is that we are not inserting a 90 bar oxygen into the sphere at 462' C before it leaves earth so that its sealed tight. We know that the gas will shrink when cold and pressurize the sphere as temperature inside rises. Which means there is near mars pressure when filled at room temperature before it gets heated.
Now I gave the thickness of the glass windows that see the opposite and its lots thicker than 2mm in the above post to keep from braking. We will need the ALON to be thicker than 2mm just at filling so as to keep it from cracking before it leaves earth. That thickness will mean that the atmosphere inside can expand and will not break it as the temperature rises when properly filled.
https://www.mwmresearchgroup.org/the-sc … art-1.html
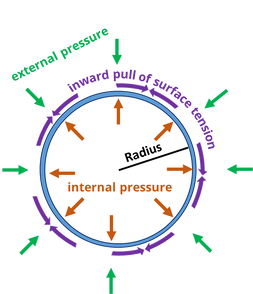
With that we are taking the tensile strength of ALON and with increasing or near zero starting have it not break.
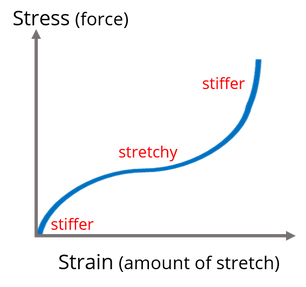
Fracture toughness 2.0 MPa·m1/2 https://en.wikipedia.org/wiki/Fracture_toughness
Flexural strength 0.38–0.7 GPa https://en.wikipedia.org/wiki/Flexural_strength
Compressive strength 2.68 GPa Stress-Strain curve https://en.wikipedia.org/wiki/Compressive_strength
Offline
Like button can go here
#49 2022-11-24 09:28:07
- tahanson43206
- Moderator
- Registered: 2018-04-27
- Posts: 23,131
Re: Balloon to survive at ground level in Atmosphere of Venus
For SpaceNut re #48
Thanks for continuing to help to move this promising topic along.
You've brought focus upon the earlier phases of the life of the probe we are discussing.
I've been focused upon the situation at the job site, where the pressure inside AND OUTSIDE the wall of the shell is 90 bar. At the job site there is NO pressure difference between the inside and the outside.
At the laboratory where the probe will be assembled, there will be NO pressure difference. The room will be a clean room, and only air of great purity will be in contact with the probe. The piece that seems to be missing from the conceptual design is the valve that allows gas to move easily between the inside and the outside.
On the job site, the valve will admit CO2 to a mechanism to separate CO from the CO2, leaving O2 to refill the interior of the probe if slow leakage occurs. The CO can be used for propulsion.
On the way to the job site, the open valve will allow all the pure air from the laboratory to bleed out to space.
During the flight to Venus, there will be vacuum inside and outside the envelope.
The valve will be open, to allow all the pure air to escape to the Universe.
Upon arrival at Venus, a supply of oxygen will be admitted to the envelope via the valve.
The supply of oxygen will be precisely matched to the external conditions, so that at NO time will a pressure difference exist between the inside and the outside.
A thickness of about 1 mm for the wall of the envelope seems about right (to me at least) if the thickness of ordinary aluminum foil is taken into account.
The wall of the envelope has three functions:
1) Keep oxygen molecules inside
2) Keep CO2 molecules outside
3) Support 17 kilograms of mass of the entire package, including envelope, frame if any and the instruments and battery.
What I am hoping we can figure out in the next few days is the mass of the shell.
If we buy a package of aluminum foil, and wrap it around a 1.24 meter diameter sphere, then we can have a fairly accurate measure of the amount of mass that will be needed.
ALON may be slightly heavier than pure aluminum, since it is an alloy.
The answer should be available in the next few days.
However, in the mean time, you have identified a crucial element of the plan ... how to protect a fragile object such as the one we are describing during flight and deployment.
The answer would seem (to me at least) to lie along the lines you've been describing. A robust, shock proof package able to protect the fragile balloon and instruments from the rigors of space launch, flight, deceleration, descent and deployment is needed. It seems to me (as i read your posts) that you are thinking along those lines.
The protective package would open upon arrival at the surface of Venus, and the instrument package in it's ALON balloon would float free.
(th)
Offline
Like button can go here
#50 2022-11-24 09:38:16
- SpaceNut
- Administrator
- From: New Hampshire
- Registered: 2004-07-22
- Posts: 29,780
Re: Balloon to survive at ground level in Atmosphere of Venus
Offline
Like button can go here